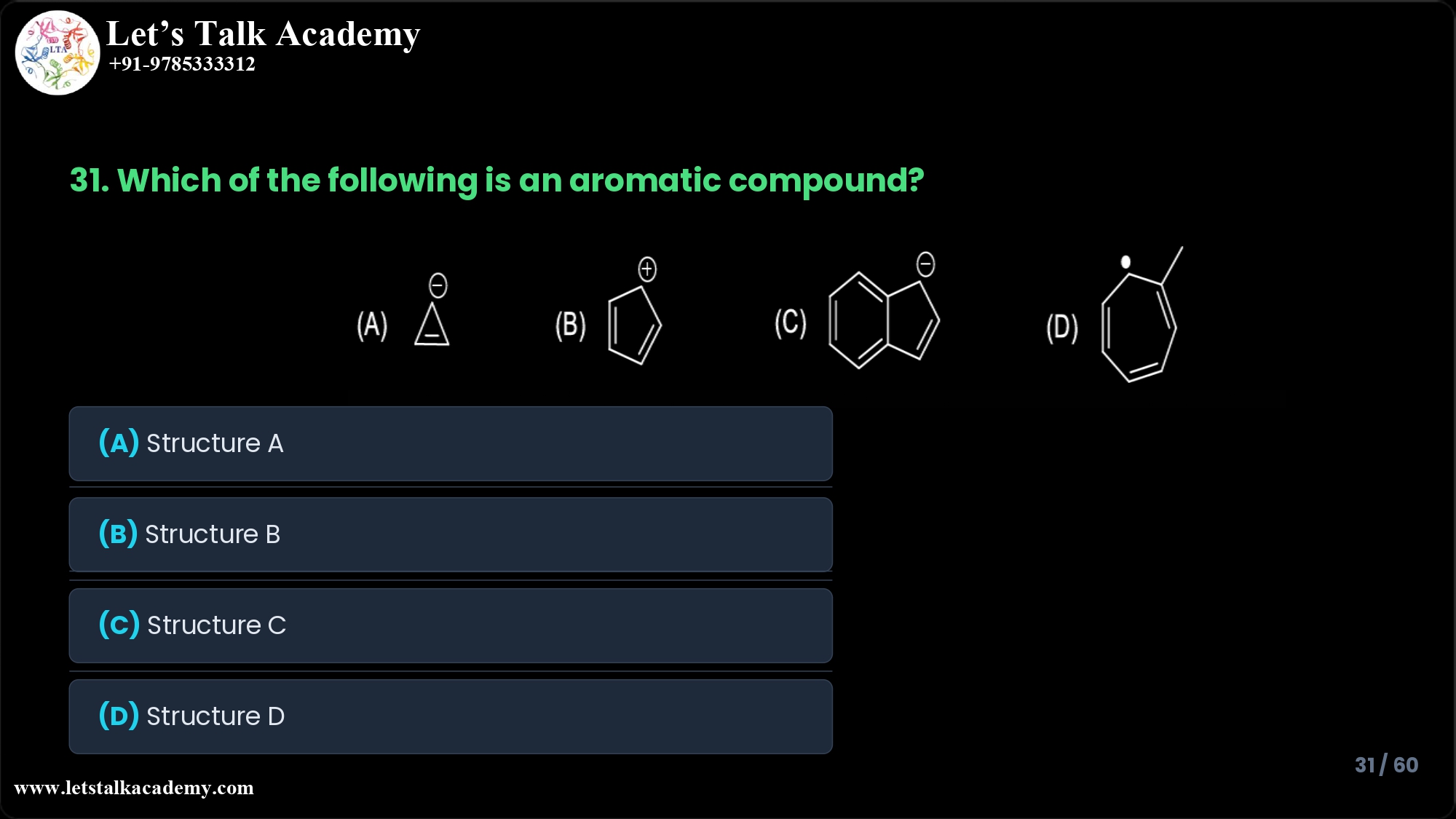
31. Which of the following is an aromatic compound?
The aromatic compound in this question is Structure C (the fused bicyclic anion with three C=C bonds and one negative charge).
Introduction
Identifying which of the following is an aromatic compound is a classic conceptual question in organic chemistry and is highly relevant for competitive exams. To solve it correctly, one must apply Hückel’s rule, check planarity, and analyze delocalization of π electrons for each given structure.
Concept recap: criteria for aromaticity
A compound is aromatic when:
-
It is cyclic and planar so that all p orbitals can overlap.
-
It is fully conjugated (every atom in the ring has a p orbital or an empty/filled orbital that can participate in delocalization).
-
It follows Hückel’s rule with 4n+2 π electrons (where n=0,1,2,…).
If a cyclic, conjugated system instead has 4n π electrons, it is antiaromatic and therefore highly unstable. If conjugation or planarity is broken, the system becomes non‑aromatic.
Option A: Three‑membered ring with negative charge
Structure A is a three‑membered ring (cyclopropenyl anion) with a negative charge shown above the ring.
-
In this ring, there are two C=C π electrons (one double bond) plus two additional electrons from the negative charge, giving a total of 4 π electrons.
-
The ring is small and can remain nearly planar, and the p orbitals can overlap, so it behaves like a cyclic conjugated system with 4 π electrons.
Because it has 4 π electrons, it satisfies the 4n pattern (with n=1) and is therefore antiaromatic, not aromatic.
Conclusion for A: Structure A is antiaromatic, hence not the correct answer.
Option B: Five‑membered ring with positive charge
Structure B is a five‑membered ring (cyclopentadienyl cation) with one double bond drawn and a positive charge.
-
For a cyclopentadienyl cation, the formal π-electron count is 4 π electrons in the ring (two C=C bonds) and an empty p orbital at the positively charged carbon.
-
The ring can be planar and conjugated, so the electrons can delocalize around the ring.
However, with 4 π electrons in a cyclic, planar, fully conjugated system, the cation falls into the 4n π-electron category and is antiaromatic. Because antiaromatic species are very unstable, the molecule avoids aromatic stabilization.
Conclusion for B: Structure B is antiaromatic, thus not the aromatic compound.
Option C: Fused bicyclic ring with negative charge (correct answer)
Structure C shows two fused rings with three double bonds overall and a negative charge on the five‑membered portion. This corresponds to an extended conjugated anion (commonly represented as a fused bicyclic anion with a 10‑π-electron system).
-
Count the π electrons:
-
Each of the three C=C bonds contributes 2 π electrons, giving 6 π from the double bonds.
-
The negative charge contributes 2 more π electrons in a p orbital.
-
Additional conjugation over the fused system allows a total of 10 delocalized π electrons in the ring framework.
-
-
The fused system can adopt a planar, fully conjugated structure, letting all p orbitals overlap and form a continuous ring of delocalized electrons.
The key is that 10 π electrons fit Hückel’s rule:
4n+2=10⇒n=2
So the bicyclic anion has a 4n+2 π-electron count, is fully conjugated and planar, and is therefore aromatic and stabilized.
Conclusion for C: Structure C is aromatic, so this is the correct choice.
Option D: Six‑membered ring with heteroatom substituent
Structure D is a six‑membered ring with one C=C bond and a heteroatom (likely O or N) attached to the ring along with a side chain (e.g., a methyl group).
-
Only one double bond appears in the ring, so there are 2 π electrons localized in one area.
-
The rest of the ring is saturated (contains sp3 carbons), breaking conjugation. The heteroatom’s lone pair is drawn outside the ring and is not clearly participating in continuous conjugation across all ring atoms.
-
Because the ring is not fully conjugated around the cycle, it cannot form a continuous loop of delocalized π electrons.
Thus, even if the ring can be nearly planar, lack of full conjugation means the system is non‑aromatic.
Conclusion for D: Structure D is non‑aromatic, so it is not the aromatic compound in the question.
Summary table: aromaticity of all options
| Structure | Ring description | π-electron count (effective) | Conjugation & planarity | Aromaticity result |
|---|---|---|---|---|
| A | Cyclopropenyl anion | 4 π electrons | Conjugated and planar | Antiaromatic |
| B | Cyclopentadienyl cation | 4 π electrons | Conjugated and planar | Antiaromatic |
| C | Fused bicyclic anion (three C=C, one charge) | 10 π electrons | Fully conjugated, planar | Aromatic (correct) |
| D | Six‑membered ring with single C=C, heteroatom | Insufficient conjugation | Not fully conjugated | Non‑aromatic |
By counting π electrons, checking planarity, and confirming full conjugation, the correct answer to “Which of the following is an aromatic compound?” in this question is Structure C.