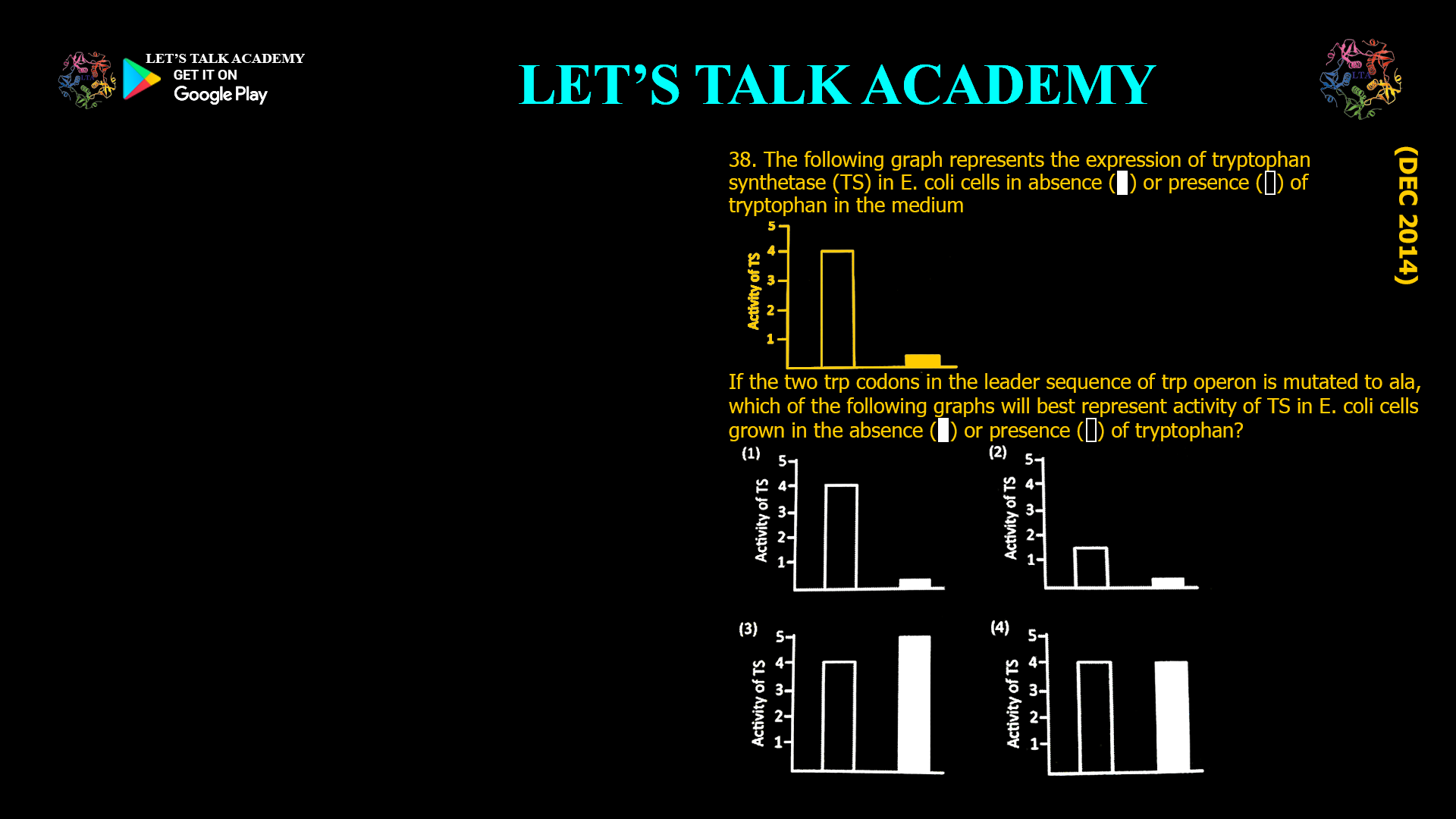
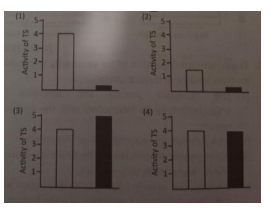
38. The following graph represents the
expression of tryptophan synthetase (TS) in E.
coli cells in absence ( ) or presence ( ) of
tryptophan in the medium
If the two trp codons in the leader sequence
of trp operon is mutated to ala, which of the
following graphs will best represent activity of
TS in E. coli cells grown in the absence ( )or presence ( ) of tryptophan?
The regulation of tryptophan biosynthesis in Escherichia coli is a paradigm of bacterial gene control, integrating transcriptional, translational, and allosteric mechanisms. Central to this regulation is the trp operon, which encodes enzymes for tryptophan synthesis, including tryptophan synthetase (TS). The operon is finely tuned by both repression and attenuation, with the leader sequence (trpL) playing a critical role in the latter. This article explores how mutation of the tandem trp codons in the trpL to alanine codons affects TS activity in E. coli and provides guidance for interpreting the corresponding activity graphs.
Regulation of the trp Operon: Repression and Attenuation
The trp operon in E. coli consists of five structural genes (trpE, trpD, trpC, trpB, trpA) that encode enzymes for tryptophan biosynthesis. The operon is regulated at two main levels:
-
Repression: When tryptophan is abundant, it binds to the trp repressor, which then blocks transcription initiation at the operator, reducing operon expression87.
-
Attenuation: Even when transcription is initiated, the leader sequence (trpL) contains regulatory elements that can cause premature termination of transcription (attenuation) if tryptophan is present. This is mediated by the coupling of transcription and translation and the formation of alternative mRNA secondary structures85.
Role of the Leader Sequence and trp Codons
The leader sequence of the trp operon contains four regions (1–4) that can form alternative secondary structures. Within the leader, there is a short open reading frame with two consecutive tryptophan codons. The ribosome’s ability to translate these codons is directly influenced by the availability of charged tryptophan tRNA.
-
High Tryptophan: The ribosome quickly translates the leader peptide, blocking the formation of the antiterminator (2–3 pairing) and allowing the terminator (3–4 pairing) to form, which terminates transcription early.
-
Low Tryptophan: The ribosome stalls at the tryptophan codons, allowing the antiterminator to form, which prevents termination and allows full transcription of the operon.
Effect of Mutating trp Codons to ala Codons
If the two trp codons in the leader sequence are mutated to alanine (ala) codons, the ribosome will no longer stall at these positions, regardless of tryptophan availability. This is because alanine is not limiting in the cell, and charged alanine tRNA is generally abundant.
Consequences of the Mutation:
-
Loss of Attenuation Control: The ribosome will always translate the leader peptide quickly, even when tryptophan is scarce. This means the antiterminator structure (2–3 pairing) cannot form, and the terminator (3–4 pairing) will always be favored, leading to premature termination of transcription.
-
Reduced Operon Expression: The trp operon will be expressed at a low level in both the presence and absence of tryptophan, because attenuation will always occur, terminating transcription before the structural genes are transcribed.
Interpretation of TS Activity Graphs
The question describes a graph representing tryptophan synthetase (TS) activity in E. coli in the absence or presence of tryptophan. For wild-type cells:
-
Absence of Tryptophan: TS activity is high because the operon is derepressed and attenuation is bypassed (ribosome stalls at trp codons, antiterminator forms).
-
Presence of Tryptophan: TS activity is low because the operon is repressed and attenuation is active (ribosome does not stall, terminator forms).
For cells with the trp codons mutated to ala codons:
-
Absence of Tryptophan: TS activity is low because attenuation is always active (ribosome does not stall, terminator forms).
-
Presence of Tryptophan: TS activity is also low, for the same reason.
Graph Interpretation:
-
Wild-type: High TS activity in the absence of tryptophan, low in its presence.
-
Mutant (trp to ala): Low TS activity in both conditions.
Biological Significance
The mutation of the trp codons in the leader sequence disrupts the ability of the cell to sense tryptophan levels via attenuation. As a result, the cell loses the ability to upregulate tryptophan synthesis when tryptophan is scarce, leading to inefficient use of resources and reduced adaptability to changing environmental conditions.
Practical Implications
Understanding the impact of leader sequence mutations is important for genetic engineering and synthetic biology. By manipulating the leader sequence, scientists can control operon expression and optimize the production of desired metabolites. However, unintended mutations that disrupt attenuation can lead to suboptimal expression and reduced yields.
Conclusion
In summary, mutation of the tandem trp codons in the trp operon leader sequence to ala codons results in constitutive attenuation, causing tryptophan synthetase activity to be low in both the presence and absence of tryptophan. The corresponding graph would show low TS activity in both conditions, unlike the wild-type, which shows high activity in the absence of tryptophan and low activity in its presence.
Key Takeaway:
When the trp codons in the leader sequence are mutated to ala codons, tryptophan synthetase activity remains low regardless of tryptophan availability, because attenuation is always active and transcription is prematurely terminated before the structural genes are expressed.
This article explains how mutations in the trp operon leader sequence affect tryptophan synthetase activity and provides a clear interpretation of the expected activity graphs for wild-type and mutant E. coli cells.