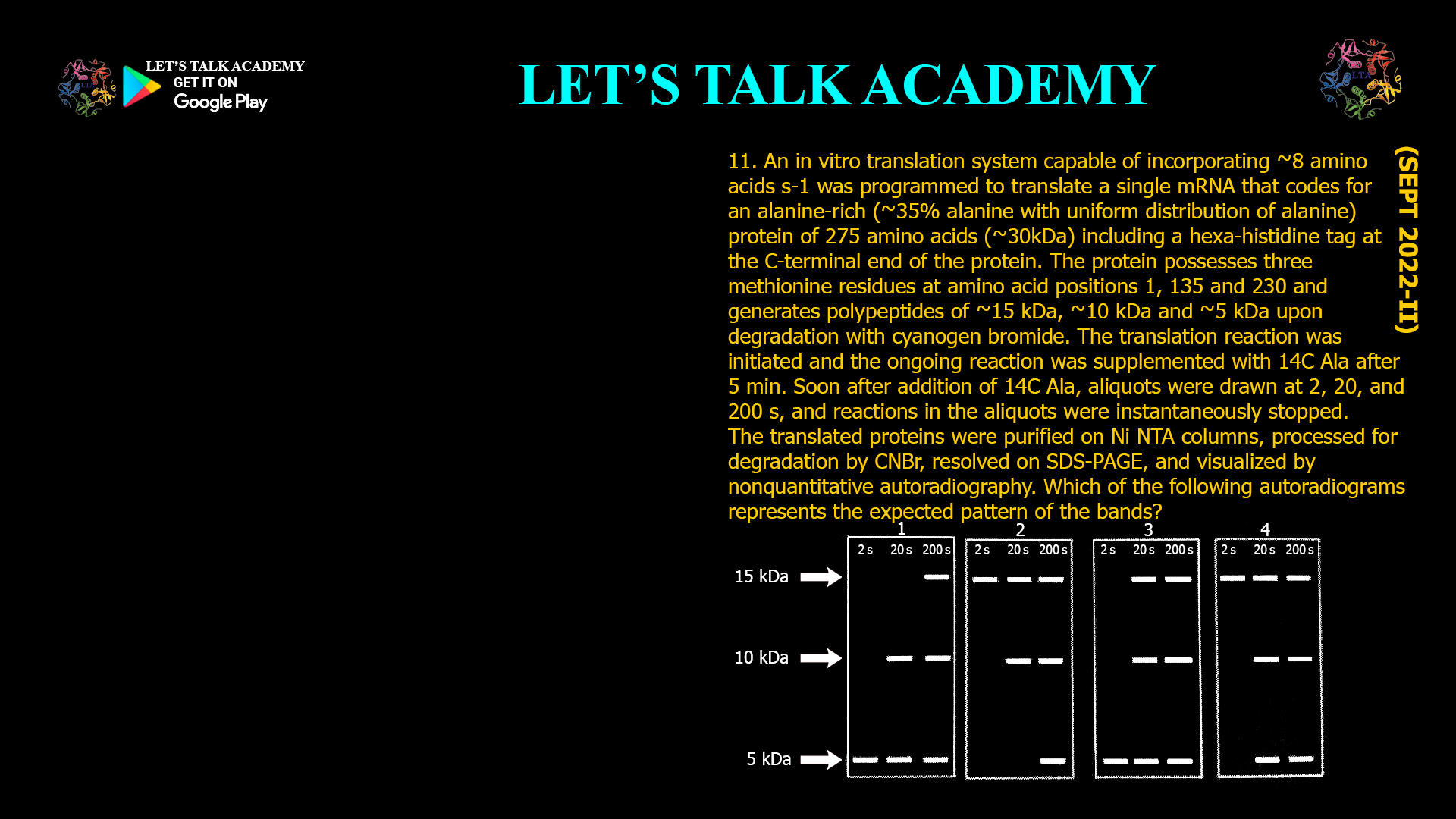
11. An in vitro translation system capable of incorporating ~8 amino acids was programmed to translate a single mRNA that codes for an alanine-rich (35% alanine with uniform distribution of alanine) protein of 275 amino acids (~30kDa) including a hexa-histidine tag at the c-terminal end of the protein. The protein possesses three methionine residues at amino acid positions 1, 135 and 230 and generates polypeptides of ~15 kDa, ~10 kDa and ~5 kDa upon degradation with cyanogen bromide. The translation reaction was initiated and the ongoing reaction was supplemented with 14C Ala after 5 min. Soon after addition of 14C Ala, aliquots were drawn at 2, 20, and 200 s, and reactions in the aliquots were instantaneously stopped. The translated proteins were purified on Ni NTA columns, processed for degradation by CNBr, resolved on SDS-PAGE, and visualized by nonquantitative autoradiography. Which Of the following autoradiograms represents the expected pattern of the bands
Introduction
In vitro translation systems are powerful tools for studying protein synthesis and processing. When combined with radiolabeled amino acids and specific cleavage agents like cyanogen bromide (CNBr), they allow detailed analysis of nascent polypeptides. Autoradiography of SDS-PAGE gels reveals the incorporation of radiolabels into protein fragments, providing insights into translation kinetics and protein structure.
This article explains why, in an experiment where ^14C-alanine is added during ongoing translation of an alanine-rich protein cleaved by CNBr, the third autoradiogram pattern among options best represents the expected results. We discuss the underlying molecular biology, cleavage specifics, and timing of radiolabel incorporation.
Experimental Context
-
Protein: 275 amino acids, ~30 kDa, with 35% alanine uniformly distributed, and a C-terminal hexa-histidine tag.
-
Methionine residues: Three methionines at positions 1, 135, and 230.
-
CNBr cleavage: Cleaves at methionine residues, producing three fragments approximately 15 kDa, 10 kDa, and 5 kDa.
-
Translation: Initiated, then supplemented with ^14C-alanine after 5 minutes.
-
Sampling: Aliquots taken at 2, 20, and 200 seconds post ^14C-alanine addition.
-
Analysis: Ni-NTA purification (selects His-tagged proteins), CNBr cleavage, SDS-PAGE, and autoradiography.
Key Principles to Understand the Expected Autoradiogram
1. Timing of Radiolabel Incorporation
-
The protein is being synthesized at ~8 amino acids per second.
-
^14C-alanine is added 5 minutes after translation starts, so only nascent peptides synthesized after this time incorporate the label.
-
Earlier synthesized polypeptides lack radiolabel and are not detected by autoradiography.
2. Distribution of Alanine Residues
-
Alanine is 35% of the amino acids, uniformly distributed.
-
Radiolabeled alanine incorporation is proportional to the length of the nascent chain synthesized after ^14C-alanine addition.
3. CNBr Cleavage Specificity
-
CNBr cleaves at methionine residues, producing three fragments:
-
Fragment 1: N-terminal to Met 135 (~15 kDa)
-
Fragment 2: Between Met 135 and Met 230 (~10 kDa)
-
Fragment 3: C-terminal fragment (~5 kDa), includes His-tag
-
4. Ni-NTA Purification
-
Only fragments containing the C-terminal His-tag (Fragment 3) or associated with it will be purified.
-
Fragments lacking the His-tag are typically lost during purification.
Why the Third Autoradiogram Pattern Is Expected
-
Since the His-tag is at the C-terminus (in Fragment 3), only the C-terminal fragment and any fragments covalently linked to it will be purified and detected.
-
At early time points (2 seconds), only very short nascent chains incorporate ^14C-alanine, resulting in faint or no bands.
-
At 20 and 200 seconds, progressively longer nascent chains incorporate more label, increasing signal intensity.
-
The autoradiogram will show bands corresponding to the C-terminal fragment (~5 kDa) and possibly larger fragments if nascent chains are incomplete or partially cleaved.
-
The first two fragments (~15 kDa and ~10 kDa), lacking the His-tag, will not be purified and thus not visible.
-
Therefore, the autoradiogram will predominantly display bands corresponding to the C-terminal fragment, matching the third pattern.
Summary Table of Expected Observations
| Fragment | Approximate Size | Contains His-tag? | Detected in Autoradiogram? | Reason |
|---|---|---|---|---|
| Fragment 1 (N-term) | ~15 kDa | No | No | Lost during Ni-NTA purification |
| Fragment 2 (Mid) | ~10 kDa | No | No | Lost during Ni-NTA purification |
| Fragment 3 (C-term) | ~5 kDa | Yes | Yes | Purified via His-tag |
Conclusion
The third autoradiogram pattern correctly reflects the expected outcome of this in vitro translation experiment with CNBr cleavage and ^14C-alanine labeling. It shows bands corresponding to the C-terminal His-tagged fragment, whose radiolabel incorporation increases over time after ^14C-alanine addition. This pattern aligns with the biochemical properties of CNBr cleavage, His-tag purification, and translation kinetics.