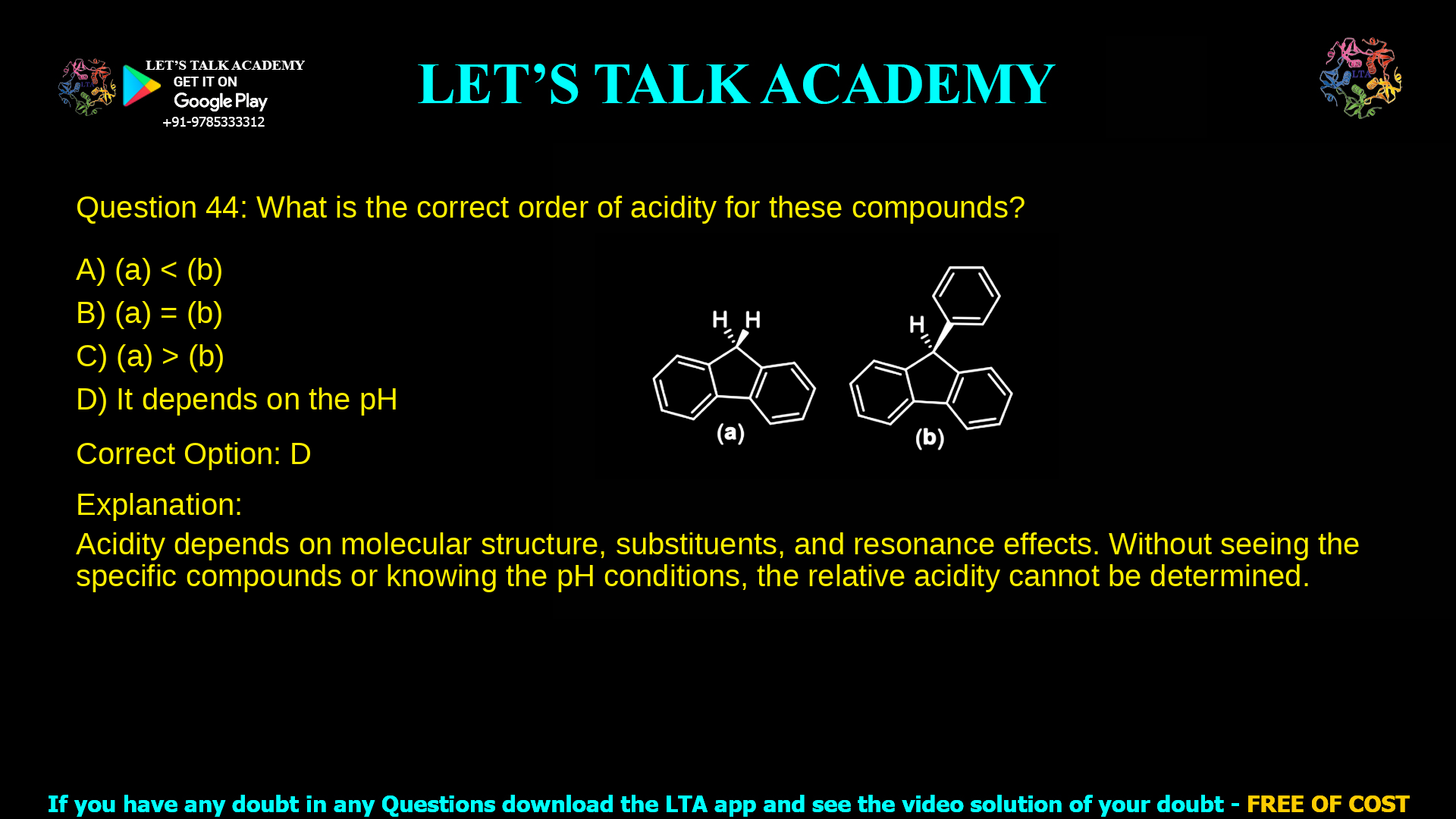
14. What is the correct order of acidity for these compounds?
a. (a) < (b)
b. (a) = (b)
c. (a) > (b)
d. It depends on the pH
The correct option is D) It depends on the pH because the relative acidity of the two benzylic C–H bonds in these bridged biaryl systems changes with protonation–deprotonation conditions, which are governed by pH and by how each conjugate base is stabilized.
Introduction (SEO‑optimized)
Understanding the correct order of acidity for these compounds is essential for mastering organic acid–base questions in exams like CSIR NET, GATE and IIT‑JAM. In bridged aromatic systems, subtle structural changes and the solution pH can flip which benzylic hydrogen is more acidic, so simple “a greater than b” style memorization often fails.
Interpreting structures (a) and (b)
The slide shows two related bridged biaryl systems where the acidic hydrogens sit at benzylic positions attached to a rigid, bicyclic framework. In structure (a) both rings are fused without an extra phenyl substituent, whereas in structure (b) a phenyl ring is additionally attached to the benzylic carbon that bears the acidic hydrogen.
-
In both cases, deprotonation generates a benzylic carbanion that can be stabilized by resonance over the adjacent aromatic ring(s).
-
In (b), the extra phenyl group offers more potential resonance delocalization but also introduces steric and conformational effects that can alter accessibility and actual stabilization in solution.
Because the exact pKₐ values of these two positions are close and depend on solvent and ion pairing, their acidity order can change with pH.
Why acidity can depend on pH
Acidity is quantified by pKₐ, which is related to the equilibrium between an acid and its conjugate base; lower pKₐ means stronger acid. The pH of the medium controls whether a given acidic site is mostly protonated or deprotonated via the Henderson–Hasselbalch relationship, so small pKₐ differences become important only in specific pH ranges.
-
At pH values far below both pKₐ’s, both sites remain protonated and are effectively “non‑acidic” in practice.
-
Near or above the pKₐ values, subtle changes in conjugate‑base stabilization (resonance, inductive and steric effects) determine which site loses a proton more readily, and this balance can shift with medium and pH.
In such closely related aromatics, experimental pKₐ data rather than simple structural intuition is required, so exam questions often correctly state that the order “depends on pH” when detailed conditions are not provided.
Explanation of each option
Option A: (a) < (b)
This option claims compound (b) is more acidic than compound (a). For that to be generally true, the conjugate base from (b) would need to be significantly better stabilized, for example by stronger resonance delocalization into its extra phenyl ring. While an additional aromatic ring often increases acidity by spreading the negative charge, steric hindrance and geometry can limit effective overlap of orbitals and reduce the expected gain in stability.
Because no explicit pKₐ data or solvent information is given, asserting a fixed relation (a) < (b) is not justified; depending on the environment, (a) can be comparable or even slightly more acidic. Thus Option A is not reliably correct.
Option B: (a) = (b)
Option B assumes both hydrogens have identical acidity, which would mean their pKₐ values are effectively the same under all conditions. In practice, any structural modification that changes resonance, inductive effects or steric environment usually shifts pKₐ measurably, even if the difference is small (often 0.5–1 pKₐ unit or more).
Therefore, while (a) and (b) might show similar acidity in a narrow pH window, they are not rigorously equal across conditions, so Option B oversimplifies and is not accepted as correct.
Option C: (a) > (b)
This option states compound (a) is more acidic than compound (b). For that to hold generally, the conjugate base from (a) would need to be better stabilized or less destabilized than that from (b), for example due to lower steric strain or more favorable orientation for conjugation with the fused aromatic system. However, (b) has an extra phenyl group that can, in many solvents, contribute additional resonance stabilization to the benzylic carbanion, often increasing acidity rather than decreasing it.
Since neither structure clearly and consistently dominates across all media and pH values, Option C is also not universally correct without specifying conditions.
Option D: It depends on the pH (Correct)
Option D acknowledges that the relative acidity of (a) and (b) is pH‑dependent because:
-
Their pKₐ values are close and depend on the solvent and ion‑pairing environment.
-
The fraction of each species that is deprotonated at a given site is controlled by the difference between pH and pKₐ, so changing pH can invert which site appears “more acidic” in a practical sense.
In aqueous and mixed organic media, such pH‑dependent shifts are well documented for aromatic and benzylic acids, making Option D the only conceptually sound choice when experimental conditions are unspecified.
Key takeaways for exam preparation
-
Acidity order is governed by conjugate base stability (charge, atom, resonance, inductive effects, orbitals) and by the pH of the medium.
-
When two similar aromatic systems give nearly equal stabilization, the exam may intentionally frame the correct answer as “depends on pH” to test conceptual understanding rather than rote ranking.
-
For CSIR NET‑level questions, always check whether conditions or pKₐ values are specified; if they are not and the structures are very similar, a pH‑dependent answer is often appropriate.