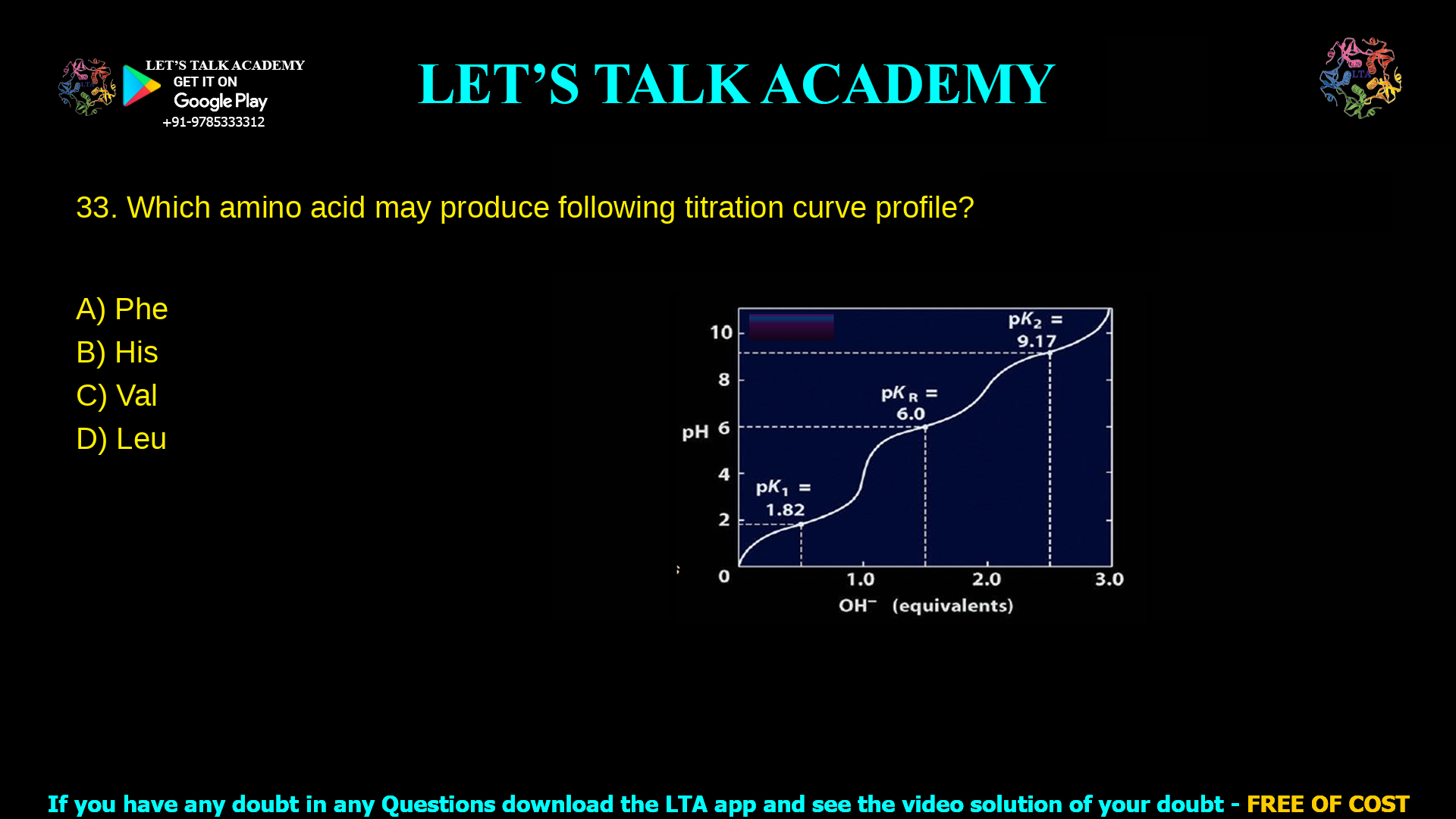
3. Which amino acid may produce following titration curve profile?
a. Phe
b. His
c. Val
d. Leu
Histidine is the amino acid that produces the given titration curve with three pKa values 1.82, 6.0 and 9.17, corresponding to the α‑COOH, imidazole side chain and α‑NH₃⁺ groups respectively.
Introduction
In amino acid titration curve questions like “which amino acid may produce following titration curve profile,” identifying the number and values of pKa steps is the fastest way to match the correct structure. The curve shown has three distinct buffering regions with pKa ≈ 1.82, 6.0 and 9.17, which is characteristic of the basic amino acid histidine and not of neutral amino acids such as phenylalanine, valine or leucine.
Reading the titration curve in the question
-
The first plateau around pH ≈ 1.8 corresponds to deprotonation of the α‑carboxyl group (pK₁ ≈ 1.8–2.0 for histidine).
-
The second plateau around pH ≈ 6.0 is due to ionization of the imidazole side‑chain (pKᵣ ≈ 6.0 for histidine).
-
The third plateau near pH ≈ 9.17 corresponds to loss of a proton from the α‑amino group (pK₂ ≈ 9.17 for histidine).
Thus the curve is triprotic and matches the known pKa set of histidine, so option B is correct.
Why option B (His) is correct
Histidine is a basic α‑amino acid with three ionizable groups: α‑COOH, α‑NH₃⁺ and an imidazole side‑chain nitrogen. Its standard pKa values in free solution are approximately 1.8 (carboxyl), 6.0 (imidazole) and 9.2 (amino), which align closely with the titration curve labels 1.82, 6.0 and 9.17 shown in the figure.
Because each titratable group produces a buffering region and half‑equivalence point, histidine’s titration curve always shows three inflection regions spanning acidic, near‑neutral, and basic pH ranges, exactly like the profile given. The isoelectric point of histidine lies between the pKa values flanking the neutral species (6.0 and 9.2), giving pI ≈ (6.0+9.2)/2≈7.6, consistent with a curve whose central region is around neutral pH.
Why the other options are incorrect
Option A: Phenylalanine (Phe)
Phenylalanine has a non‑ionizable benzyl side chain, so it behaves like a typical neutral amino acid during titration. Such amino acids show only two ionization steps (α‑COOH and α‑NH₃⁺) with pKa values around 2.2 and 9.1, so their titration curves have only two buffering plateaus, not the three seen in the question.
Because there is no extra ionizable side group, Phe cannot produce a titration curve with a third midpoint at pH ≈ 6.0, so this option does not fit the data.
Option C: Valine (Val)
Valine has a branched aliphatic side chain that is also non‑ionizable under physiological pH, giving it only two titratable groups (α‑COOH and α‑NH₃⁺) with pKa values similar to alanine or leucine. Its titration curve therefore shows two, not three, inflection points and lacks a buffering region around pH 6, so it cannot match the given profile.
Option D: Leucine (Leu)
Leucine likewise has a hydrophobic, non‑ionizable isobutyl side chain and titrates as a diprotic amino acid with only α‑carboxyl and α‑amino ionizations. The resulting titration curve contains two pKa values near 2 and 9–10 with no additional plateau, contradicting the observed third pKa at 6.0 in the question’s diagram.
Summary table: options vs titration features
| Amino acid | Side chain type | Number of ionizable groups | Typical pKa set (approx.) | Matches 1.82, 6.0, 9.17 curve? |
|---|---|---|---|---|
| Histidine | Basic imidazole | 3 (α‑COOH, α‑NH₃⁺, side‑chain) | 1.8, 6.0, 9.2 | Yes |
| Phenylalanine | Non‑ionizable aromatic | 2 (α‑COOH, α‑NH₃⁺) | 2.2, 9.1 | No |
| Valine | Non‑ionizable aliphatic | 2 (α‑COOH, α‑NH₃⁺) | ≈2.3, 9.6 | No |
| Leucine | Non‑ionizable aliphatic | 2 (α‑COOH, α‑NH₃⁺) | ≈2.4, 9.6 | No |