(DEC 2012) 70. An enzyme catalyzed reaction was measured in the presence and absence of an inhibitor for an uncompetitive inhibition, (1) only Km is increased (2) only is Vmax […]
Tag: csir biochemistry
Tag: csir biochemistry
No posts found.
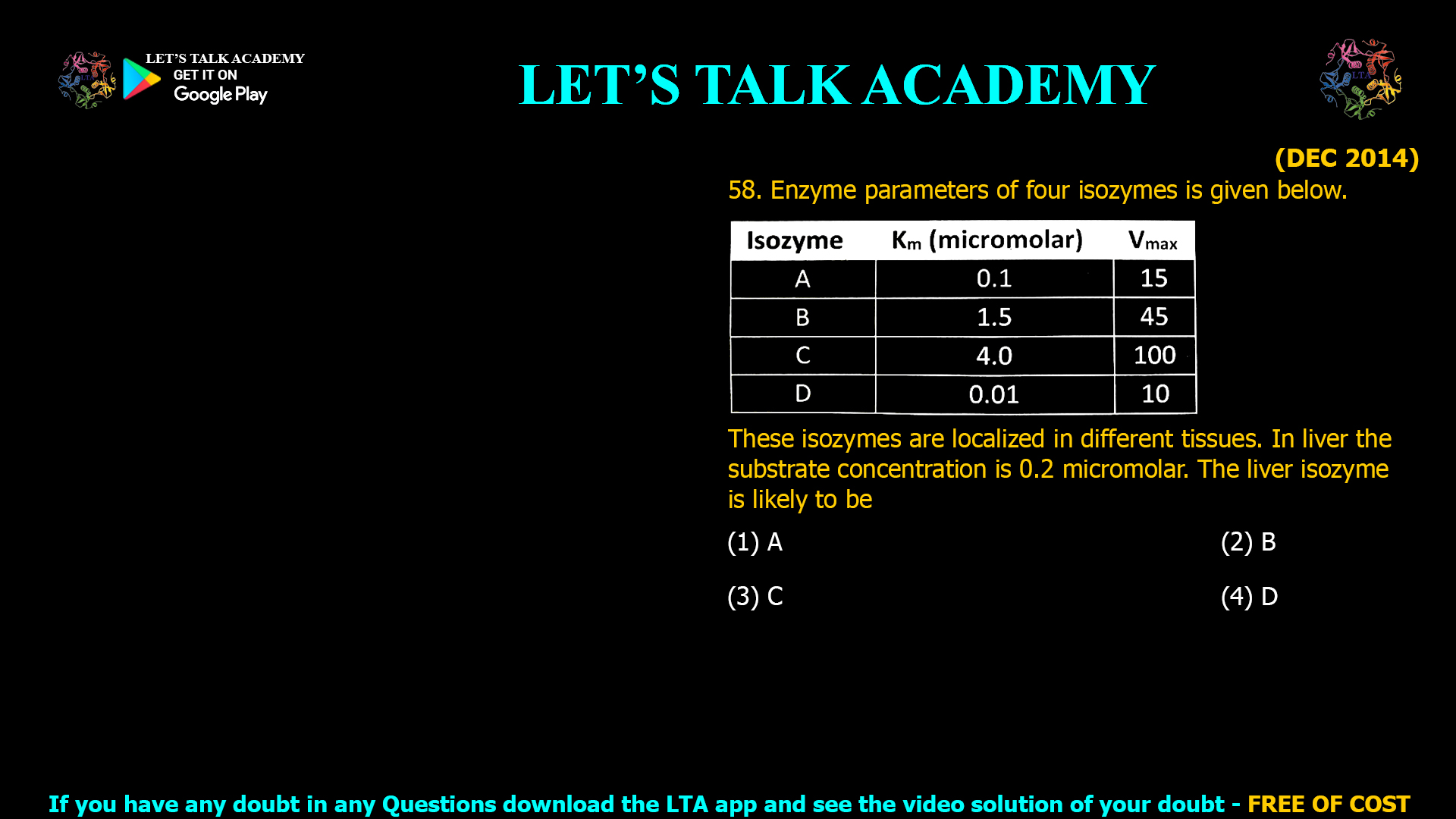
Selecting the Optimal Isozyme for Low Substrate Concentrations: Liver Enzyme Kinetics Explained
- admin
- September 12, 2025
- 33 Comments
The correct answer is (1) A. Introduction Isozymes are enzyme variants catalyzing the same reaction but optimized for different tissue environments, frequently distinguished by their kinetic properties: the Michaelis constant (KmKm) […]
Correcting Misconceptions in Enzyme Kinetics: Allosteric Behavior and Enzymatic Efficiency
- admin
- September 12, 2025
- 35 Comments
(JUNE 2019) 57. Choose the INCORRECT statement from the following statements made for an enzyme- catalyzed reaction (1) The kinetic properties of allosteric enzyme do not diverge from Michaelis-Menten behaviour. […]
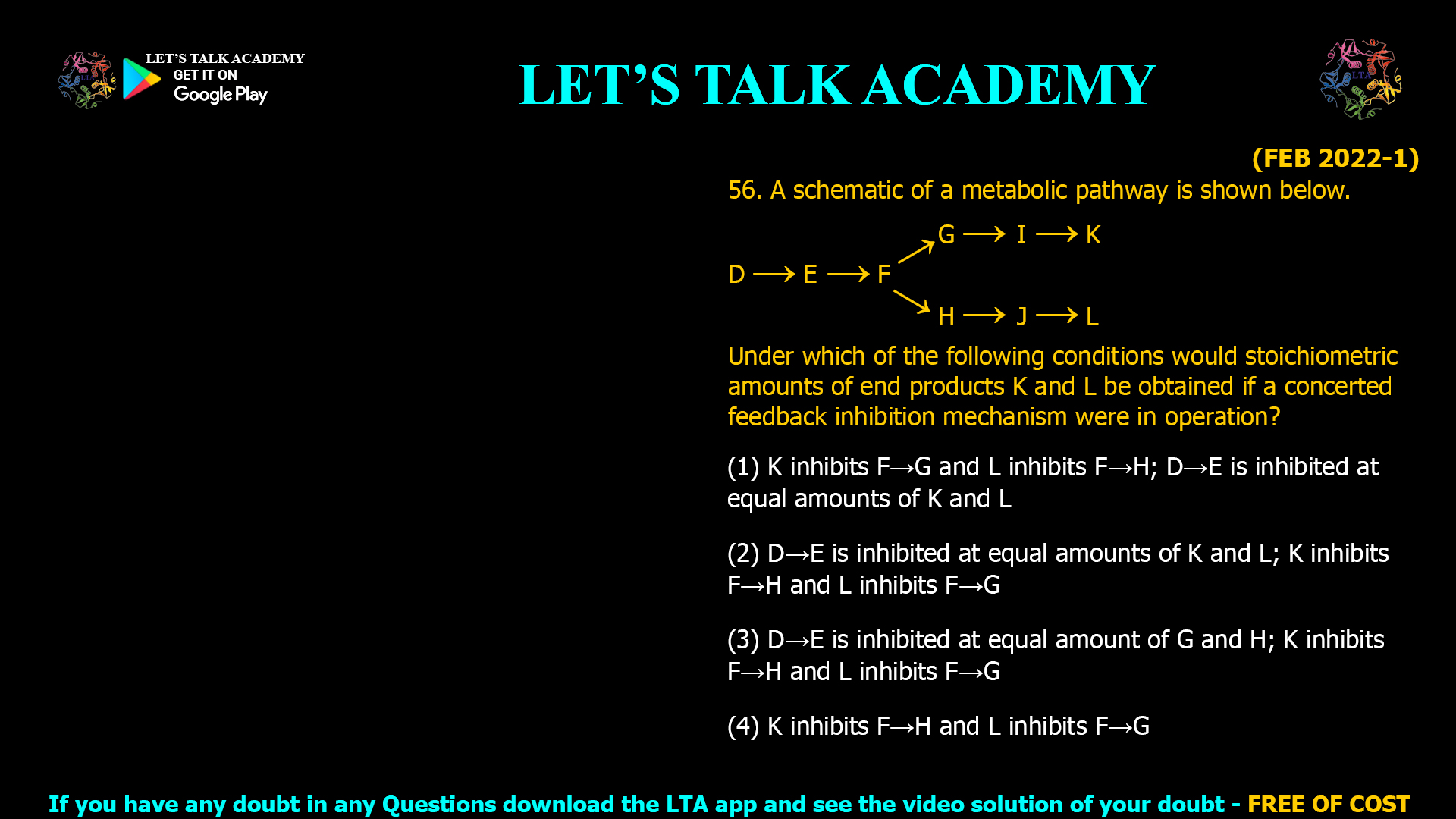
Concerted Feedback Inhibition in Metabolic Pathways: Achieving Stoichiometric End Product Balance
- admin
- September 12, 2025
- 31 Comments
The correct answer is (1) K inhibits F→G and L inhibits F→H; D→E is inhibited at equal amounts of K and L. Introduction Metabolic pathways often branch to produce multiple end […]
Understanding Allosteric Regulation: Identifying Positive and Negative Effectors Through the Allosteric Constant LL
- admin
- September 12, 2025
- 28 Comments
(DEC 2019 ASSAM) 55. An allosteric enzyme has two heterotropic effectors, X and Y. The allosteric constant, L for the enzyme in the absence of effector molecules is 180. For […]
Understanding the Hill Equation and Hill Plot: Describing Cooperative Enzyme Kinetics
- admin
- September 12, 2025
- 36 Comments
(NOV 2020-11) 53. The Hill equation and its plot describe the following enzyme kinetic behaviours A. Saturation Kinetics B. Cooperative Kinetics C. Log Vi/(Vmax — Vi) versus Log [s] D. […]
Interpreting Ligand Binding Isotherms: Cooperativity and Protein Structure
- admin
- September 12, 2025
- 29 Comments
The correct answer is (2) B is obtained with protein with positive cooperativity. Introduction The analysis of ligand binding isotherms provides insight into protein-ligand interactions, stoichiometry, and cooperative behavior in molecular […]
Analyzing Elastase Substrate Specificity Using Kinetic Data: Rate, Residue Size, and Cleavage Preferences
- admin
- September 12, 2025
- 24 Comments
The correct answer is (2) (B), (D), (E). Introduction Elastase is a proteolytic enzyme known for its specificity based on the size and nature of amino acid residues adjacent to its […]
Understanding Enzyme Kinetic Plots: Identifying Equations Without Independent Variables on Axes
- admin
- September 12, 2025
- 23 Comments
(SEPT 2022-1) 34. A student was asked to plot a graph representing enzyme kinetic data for initial velocity, vO , and substrate concentration [S] using any of the equations given […]
Substrate Concentration for Half-Maximal Velocity: Understanding Michaelis Constant (Km)
- admin
- September 12, 2025
- 49 Comments
(JUNE 2010) 27. Km for enzyme is equal to where (1) V0=Vmax (2) 2V0= Vmax (3) 4V0=Vmax (4) V0 = 2 Vmax The correct answer is (2) Introduction In enzymatic reactions, […]



![(NOV 2020-11) 53. The Hill equation and its plot describe the following enzyme kinetic behaviours A. Saturation Kinetics B. Cooperative Kinetics C. Log Vi/(Vmax — Vi) versus Log [s] D. Log (Vmax — Vi)/Vi versus Log [s]-1 Which one of the following combination represent correct descriptions? (1) A and C (2) B and C (3) B and D (4) A and D](https://www.letstalkacademy.com/wp-content/uploads/2025/09/53.jpg)


![(SEPT 2022-1) 34. A student was asked to plot a graph representing enzyme kinetic data for initial velocity, vO , and substrate concentration [S] using any of the equations given below. The student used an equation for which neither X-axis nor Y-axis had independent variables. Which one of the following equation might the student have used? (1) 1/ vO = (Km/Vmax) 1/[S]+ 1/Vmax) (2) [S]/vO = [S]/Vmaxx + (Km /Vmax) (3) vO / [S] = (Vmax / Km) - vO / Km (4) vO = V max [S] / Km + [S]](https://www.letstalkacademy.com/wp-content/uploads/2025/09/34.jpg)



