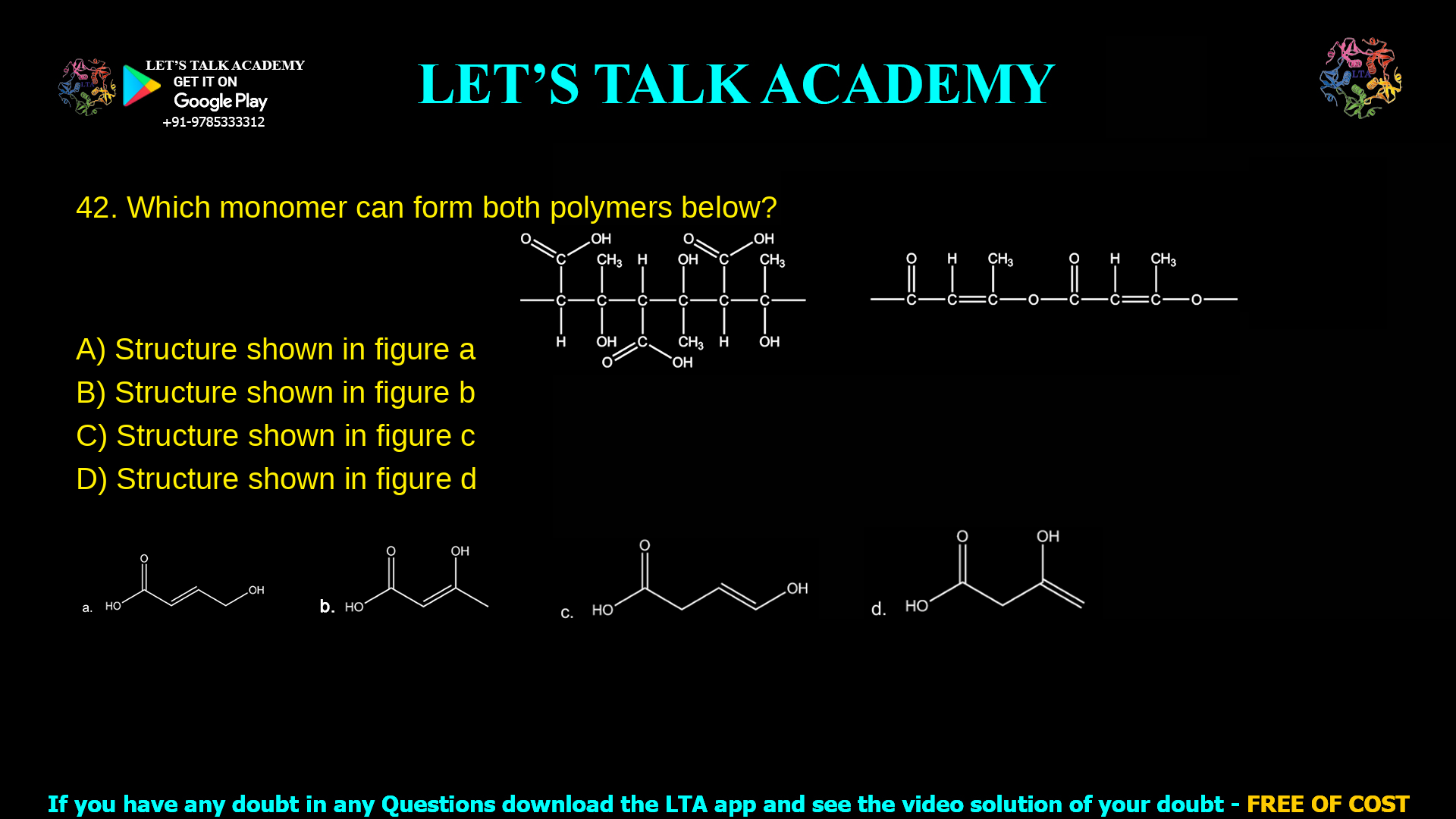
12. Which monomer can form both polymers below:
The monomer that can form both of the given polymers is the structure shown in figure d.
Question overview
The question asks: “Which monomer can form both polymers below?” The two polymers shown are:
-
A polyester formed by condensation between a carboxylic acid group and an alcohol group, giving multiple ester linkages along the chain.
-
An addition polymer formed by opening a carbon–carbon double bond of an alkene (a vinyl group) to give a long –CH–CH– backbone.
Therefore, the correct monomer must contain:
-
A carboxylic acid group −COOH
-
A hydroxyl (alcohol) group −OH
-
A carbon–carbon double bond C=C for addition polymerisation
Such molecules are often called unsaturated hydroxycarboxylic acids, which can form both polyesters (by condensation) and polyalkenes (by addition).
Option a: structure in figure a
Structure a is a molecule with:
-
One carboxylic acid group −COOH at one end.
-
One hydroxyl group −OH attached to the same carbon that is part of a carbonyl, effectively forming an enol–acid system.
However, the chain in figure a does not show a clear terminal C=C double bond separated from the –COOH and –OH groups that could undergo straightforward chain-growth addition polymerisation. Addition polymers typically require a well‑defined vinyl group such as –CH2=CH– or substituted alkenes. Because this monomer lacks a suitable isolated double bond, it can form a polyester by condensation of –COOH and –OH, but it cannot easily give the shown addition polymer backbone, so option a is incorrect.
Option b: structure in figure b
Structure b again has:
-
A carboxylic acid group.
-
A hydroxyl group on an adjacent carbon (a hydroxy acid).
But the rest of the chain ends in a saturated group (no C=C double bond), so there is no functional vinyl group available for addition polymerisation. This monomer can form a polyester by condensation, but cannot give the linear –CH–CH– backbone seen in the second polymer. Therefore, structure b cannot form both polymers and is also incorrect.
Option c: structure in figure c
Structure c shows:
-
A terminal carboxylic acid group −COOH.
-
A separated alkene (C=C) at the other end of the chain.
However, it does not contain a hydroxyl group –OH, so it is not a hydroxycarboxylic acid. Without an –OH group, it cannot undergo self‑condensation to form the ester linkages that define the first polymer. Although the vinyl end could form an addition polymer, the absence of –OH prevents polyester formation. Thus option c cannot form both polymers, so it is incorrect.
Option d: structure in figure d (correct answer)
Structure d contains all three required features in a single molecule:
-
A carboxylic acid group −COOH.
-
A hydroxyl (alcohol) group −OH.
-
A terminal carbon–carbon double bond (C=C) at the opposite end of the chain.
Because of this:
-
Formation of the first polymer (polyester):
-
The –COOH of one monomer reacts with the –OH of another monomer, eliminating water and forming an ester linkage –CO–O–.
-
Repetition of this condensation gives the polyester chain shown in the first structure, with alternating –CO–O– linkages and residual substituents matching the monomer skeleton.
-
-
Formation of the second polymer (addition polymer):
-
The C=C double bond in each monomer can undergo addition polymerisation, where the double bond opens and links to neighboring monomer units to create a long –CH–CH– backbone.
-
In this process, the –COOH and –OH groups remain as side or internal functional groups attached to the chain, matching the second polymer’s pattern of esterified units after possible intra‑ or intermolecular ester formation.
-
Because structure d is an unsaturated hydroxycarboxylic acid, it can participate in both condensation polymerisation (forming polyesters) and addition polymerisation (via its C=C bond). Therefore, option d is the only monomer capable of forming both depicted polymers.