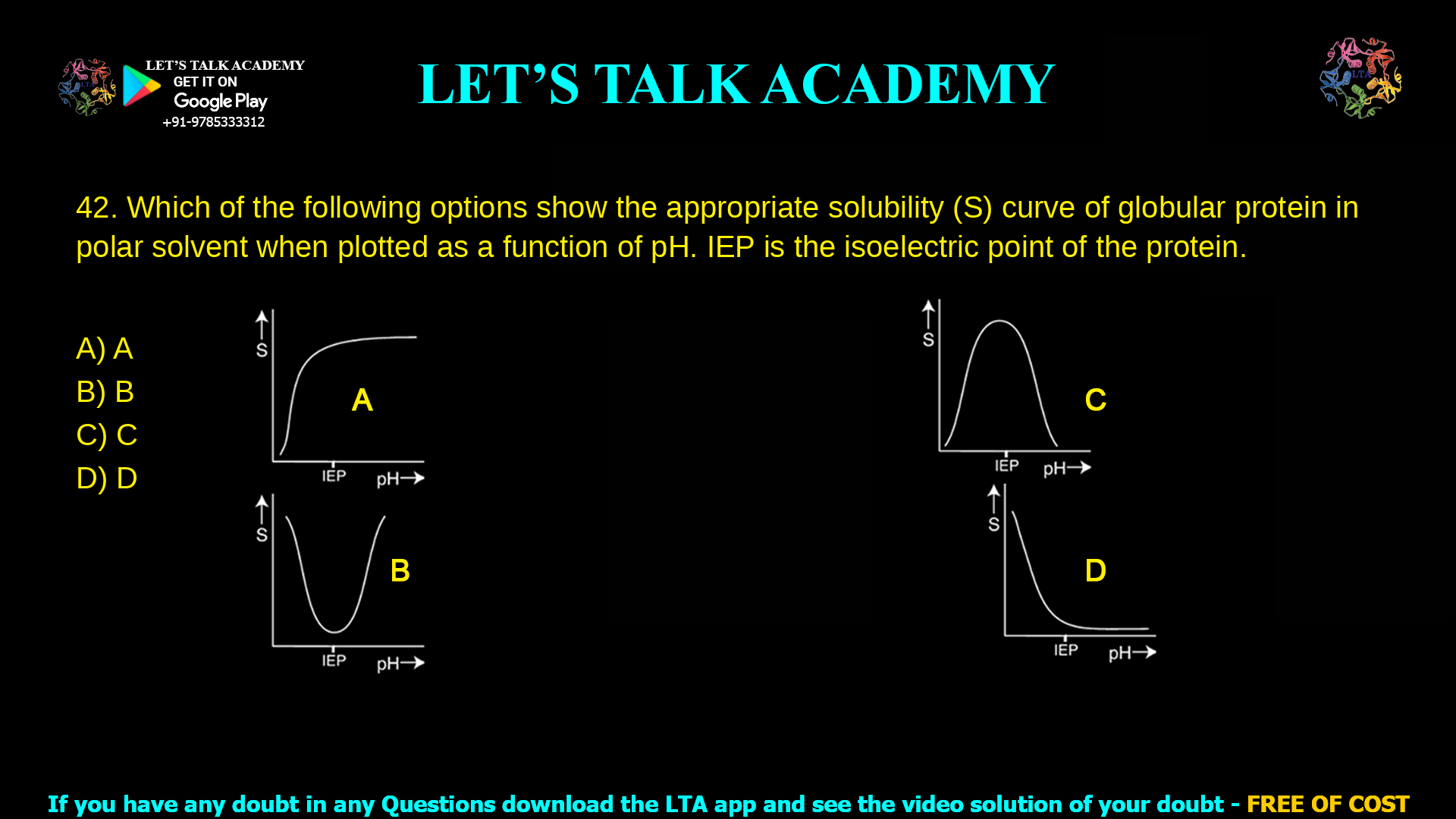
Which of the following options show the appropriate solubility (S) curve of
globular protein in polar solvent when plotted as a function of pH. IEP is the
isoelectric point of the protein.
The correct solubility–pH curve for a globular protein in a polar solvent is option B, which shows a minimum solubility at the isoelectric point (IEP) and higher solubility at pH values both below and above the IEP.
Introduction
The solubility curve of a globular protein in a polar solvent as a function of pH typically shows that solubility is lowest at the isoelectric point (pI or IEP) and increases more or less symmetrically on either side as the protein becomes positively or negatively charged. At the IEP, the protein has zero net charge, electrostatic repulsion is minimal, and molecules easily aggregate and precipitate, which is why this principle is widely used in isoelectric precipitation and salting‑out techniques.
Concept: solubility vs pH for globular proteins
-
The isoelectric point is the pH at which a protein’s net charge is zero, so attractive interactions dominate and solubility is minimal.
-
When pH moves away from pI (more acidic or more basic), the protein acquires net positive or negative charge, electrostatic repulsion between molecules increases, water interacts better with charged groups, and solubility rises, giving a U‑shaped solubility–pH curve for many globular proteins.
Analysis of each option
Option A
In option A, solubility starts low at the IEP and then increases with pH, approaching a plateau, with no increase on the acidic side.
-
This curve suggests solubility is minimal only at IEP and increases only toward higher pH, which would be true only for a restricted pH range where data are taken from slightly below to above neutrality, but it does not capture the typical behavior on both sides of pI.
-
Since the question asks for the appropriate solubility curve as a function of pH in a polar solvent, the expected answer must show low solubility at IEP and higher solubility at both lower and higher pH values, so option A is not correct.
Option B (correct)
Option B shows a U‑shaped solubility curve with the lowest point at the IEP and increased solubility at pH values below and above the IEP.
-
Experimental studies on food and whey proteins consistently report this U‑shaped profile: solubility is minimum at pI (around pH 4–6 for many proteins) and increases toward extreme acidic or basic pH.
-
This reflects the fundamental rule that proteins are least soluble at their isoelectric point and more soluble when they carry net charge, so option B correctly represents the solubility–pH relationship for a typical globular protein in a polar solvent.
Option C
Option C displays a bell‑shaped curve with maximum solubility at the IEP and lower solubility on both acidic and basic sides.
-
This is the inverse of established protein behavior; numerous studies and textbooks emphasize that solubility is minimal at pI, not maximal, because reduced net charge favors aggregation and precipitation.
-
Therefore, option C contradicts the characteristic solubility–pH pattern of globular proteins and is incorrect.
Option D
Option D shows high solubility at IEP that decreases with increasing pH, resembling a monotonically falling curve with no rise on the acidic side.
-
Such a graph would imply that the isoelectric point is the condition of greatest solubility, again opposing the experimentally observed minimum at pI for most globular proteins in aqueous polar media.
-
Because it neither shows low solubility at pI nor the U‑shaped dependence on pH, option D is also incorrect.
Why solubility is minimum at IEP
-
At pH = pI, positive and negative charges on the protein balance out, yielding zero net charge so that electrostatic repulsion between molecules is minimized; van der Waals and hydrophobic attractions then dominate, favoring close packing, aggregation and precipitation, which lowers apparent solubility.
-
At pH values away from pI, acquisition of net positive or negative charge increases repulsion, improves hydration of charged groups, and keeps protein molecules dispersed, thereby increasing solubility in the polar solvent.