The correct answer is (1) A.
Introduction
Isozymes are enzyme variants catalyzing the same reaction but optimized for different tissue environments, frequently distinguished by their kinetic properties: the Michaelis constant (Km) and the maximum velocity (Vmax). Choosing which isozyme is best suited for a specific tissue relies heavily on matching substrate concentrations with the enzyme’s affinity for its substrate. This article unpacks the rationale for isozyme selection in the liver, where the substrate concentration is especially low.
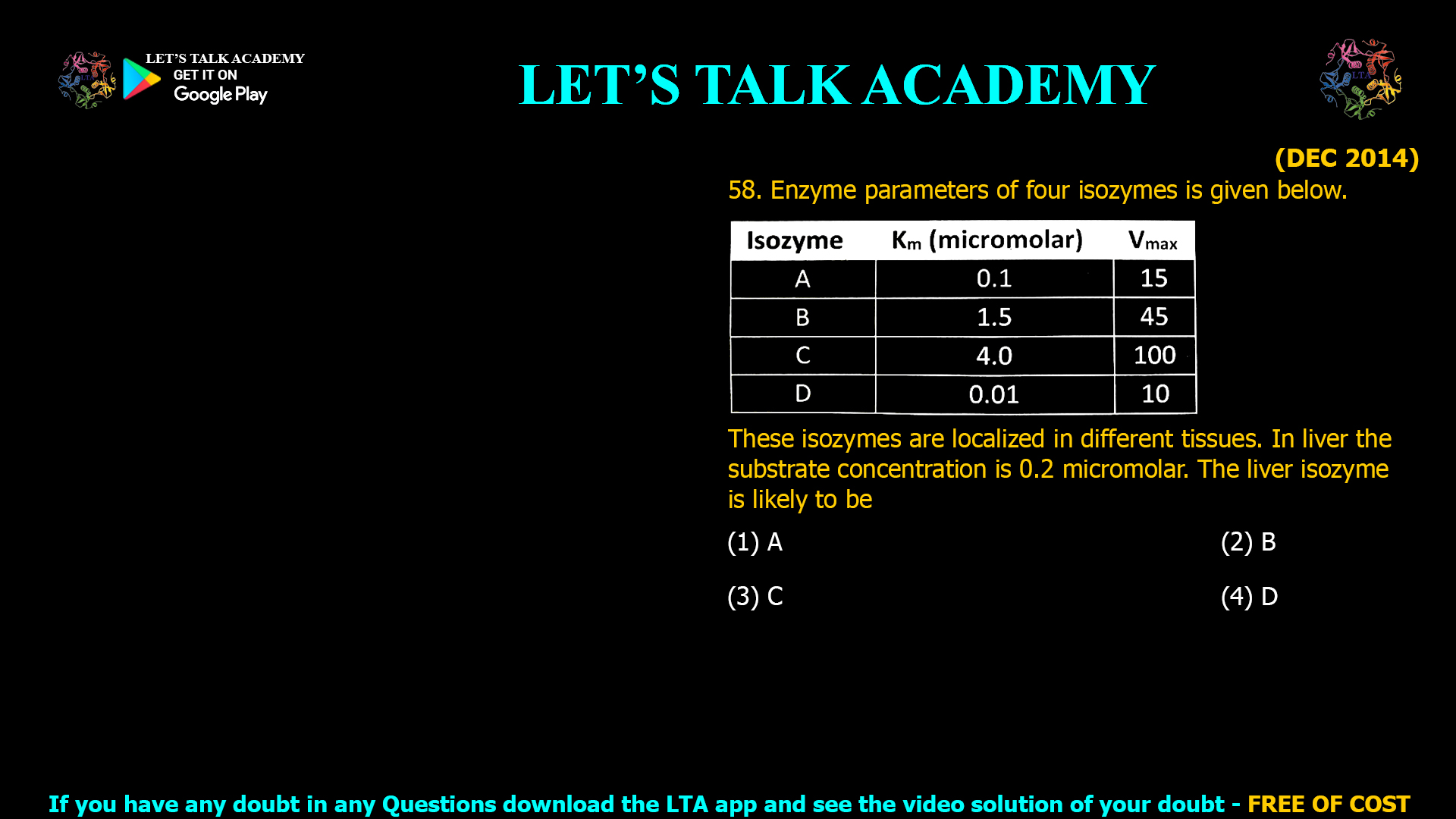
Isozyme Kinetics Table
| Isozyme | Km (µM) | Vmax |
|---|---|---|
| A | 0.1 | 15 |
| B | 1.5 | 45 |
| C | 4.0 | 100 |
| D | 0.01 | 10 |
Why is Km Important?
-
Km reflects the substrate concentration at which the enzyme works at half-maximal velocity.
-
When substrate concentration ≪Km, small changes in substrate levels strongly affect velocity.
-
Low Km means high affinity—the enzyme works efficiently even at low substrate concentrations.
Matching Isozyme to Liver Substrate Conditions
-
Liver substrate concentration: 0.2 µM
-
Isozymes most effective when their Km is close to or below tissue substrate concentration.
-
Among the given options:
-
Isozyme A: Km=0.1μM
-
Isozyme D: Km=0.01μM
-
Both have high affinity; D even higher, but its Vmax=10 (much lower than A’s 15).
-
A’s Km just below tissue level, meaning near-optimal velocity for physiological substrate levels.
-
-
B and C have much higher Km values, requiring higher substrate concentrations for efficient action—less suited for the liver’s low substrate condition.
Isozyme Activity Comparison
-
At [S]=0.2μM, both A and D will be active.
-
However, at physiological substrate concentration just above A’s Km, A provides an optimal compromise between affinity (Km) and capacity (Vmax), making it the most likely liver isozyme.
Summary Table
| Isozyme | Km (µM) | Vmax | Efficiency at [S]=0.2μM |
|---|---|---|---|
| A | 0.1 | 15 | Highest (matches tissue) |
| D | 0.01 | 10 | High affinity, lower capacity |
| B | 1.5 | 45 | Too high Km |
| C | 4.0 | 100 | Too high Km |
Conclusion
For a substrate concentration of 0.2 µM in liver tissue, Isozyme A is most likely selected due to its Km=0.1μM and reasonable Vmax, efficiently catalyzing reactions at low substrate levels. This illustrates the critical role of kinetic parameters in evolutionary enzyme specialization across different tissues.



33 Comments
Khushi Vaishnav
September 12, 20250.1 is the correct answer if substrate conc. Is 0.2 mm
Kanica Sunwalka
September 13, 2025low km – > affinity more
i.e. enzyme works efficiently even at low S conc .
liver S conc = 0.2
km – close to or below tissue conc
vmax – D ka much lower than A
so , A and D both have high affinity for liver
but A have highest
Aakansha sharma Sharma
September 13, 20250.1 is the correct answer if substrate conc. Is 0.2 mm
Rishita
September 14, 2025Option a is correct
Rishita
September 14, 2025Option a
Kajal
September 14, 2025Option A is correct answer bcz its km value is more near to the liver substrate concentration
yashika
September 14, 2025Best enzyme is the one whose km matches tge actual substrate concentration
yashika
September 14, 2025Best enzyme is the one whose km matches the actual substrate concentration
Mohd juber Ali
September 14, 2025Km inverse perportional to affinity
According question
Isozyme A and D affinity high so there km low
So isozyme A is used bcz it km is low
Anju
September 14, 2025Ans: A
Low km high affinity
A km value is more close to liver substrate concentration
Dharmpal Swami
September 14, 2025Option A is write bcoz it’s km value more near the liver substrate concentration
Pooja
September 14, 2025Option a is correct
Pallavi Ghangas
September 14, 2025A
Aafreen Khan
September 14, 2025Option 1st is correct answer because the Km value is more near to substrate concentration of liver
Soniya Shekhawat
September 14, 2025Km inverse proportional to affinity so isozyme A is very low km compare to other and km is less then enzyme affinity is high so 1st option is correct
Kirti Agarwal
September 14, 2025Km is less so affinity is high
Then enzyme work on low substrate
So correct answer is opt A
Palak Sharma
September 14, 2025Km is less so affinity will be high. Km should be 0.1 in this case.
Ajay Sharma
September 14, 2025A is correct because the km value is just below tissue substrate conc^n and Vmax is 15 , makes it more correct over d
Sakshi Kanwar
September 14, 2025A is most likely selected due to its Km value is 0.1
Priya dhakad
September 14, 20250.1 is the correct answer if substrate conc. Is 0.2 mm.
Deepika sheoran
September 14, 2025Option 1st is correct
Km inverse proportional to affinity so , isoenzyme A is very low km compair to less the enzyme affinity is high.
Anurag Giri
September 15, 2025Km inverse perportional to affinity
According question
Isozyme A and D affinity high so there km low
So isozyme A is used bcz it km is low
Roopal Sharma
September 15, 2025Low km high affinity ans A km value is close to its substrate concentration.
Bhawna Choudhary
September 15, 2025Option A is correct answer
Nilofar Khan
September 16, 2025Correct answer is A
Km is inversely proportional to affinity.
Isozymes most effective when their Km is close to tissue substrate
Payal Gaur
September 16, 2025Option 1. A km~ substrate concentration
(Km is inversely proportional to affinity then km is close to substrate concentration)
Tanvi Panwar
September 16, 2025Liver isozyme is most likely to be A bcz its km is low and Vmax. is high.
Khushi Agarwal
September 17, 2025The correct answer is (1) A..
Avni
September 17, 2025the Correct answer is 1. (A)
Priya khandal
September 17, 2025Option a is correct because km is low and vmax is high
Muskan Yadav
September 19, 20250.1 is the correct answer if substrate conc. Is 0.2 mm.
Savita Garwa
September 21, 2025Option 1st is correct answer because the Km value is more near to substrate concentration of liver.
Kajal
September 25, 2025substrate concentration of 0.2 µM in liver tissue, Isozyme A is most likely selected due to its Km=0.1μM and reasonable Vmax, efficiently catalyzing reactions at low substrate levels. This illustrates the critical role of kinetic parameters in evolutionary enzyme specialization across different tissues.