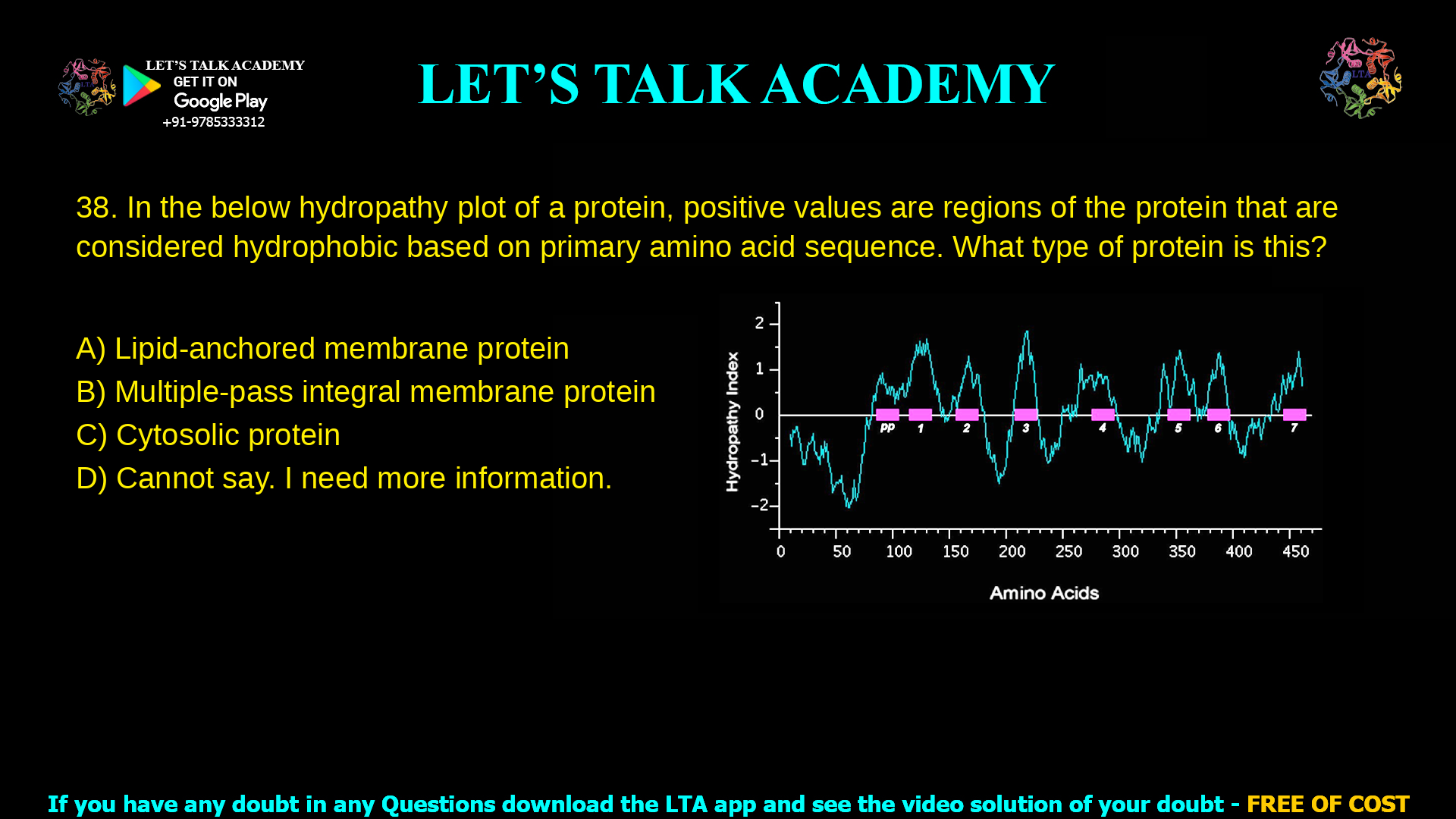
8. In the below hydropathy plot of a protein, positive values are regions of the protein
that are considered hydrophobic based on primary amino acid sequence. What type
of protein is this?
a. Lipid-anchored membrane protein
b. Multiple-pass integral membrane protein
c. Cytosolic protein
d. Cannot say. I need more information.
This hydropathy plot represents a multiple-pass integral membrane protein, because it shows several strongly hydrophobic segments corresponding to multiple transmembrane domains.
Introduction: hydropathy plot of a protein
A hydropathy plot of a protein is a graphical way to see how hydrophobic or hydrophilic each stretch of amino acids is along the polypeptide chain. Positive peaks indicate hydrophobic segments that are likely to sit in a membrane, whereas negative regions indicate hydrophilic segments exposed to water. When several long, strong hydrophobic peaks appear, the hydropathy plot of a protein usually indicates a multiple-pass integral membrane protein with many transmembrane helices.
In the given question, the plot shows seven distinct hydrophobic segments (positive regions) of around 20 residues each, typical of a receptor or transporter that crosses the bilayer many times. This pattern allows confident classification of the protein type.
Detailed analysis of the correct option (B)
Multiple-pass integral membrane proteins span the lipid bilayer several times with multiple hydrophobic α-helices, each usually about 18–25 amino acids long. On a Kyte–Doolittle hydropathy plot, each transmembrane helix appears as a broad positive peak above a threshold (commonly around 1.6) when using a window size of about 19–21 residues.
The provided hydropathy plot shows seven such hydrophobic peaks distributed along the sequence, each highlighted as a potential transmembrane segment. This is characteristic of classical seven-pass membrane proteins such as G protein–coupled receptors, which are textbook examples of multiple-pass integral membrane proteins. Therefore, the best answer is B) Multiple-pass integral membrane protein.
Why option A is incorrect: lipid-anchored membrane protein
Lipid-anchored proteins are covalently attached to lipids (such as GPI anchors, prenyl groups, or fatty acyl chains) that insert into one leaflet of the membrane, but the polypeptide chain itself does not typically cross the bilayer as a long hydrophobic helix. Their membrane association is mediated by the lipid moiety, so their amino acid sequence often lacks multiple long, strongly hydrophobic segments that would appear as repeated high peaks in a hydropathy plot.
A lipid-anchored protein might show one short hydrophobic region near the site of lipid attachment, but not seven separate, long hydrophobic segments spanning the protein’s length. Because the question’s hydropathy plot clearly displays multiple putative transmembrane segments, it does not fit the typical pattern of a lipid-anchored membrane protein, making option A incorrect.
Why option C is incorrect: cytosolic protein
Cytosolic proteins are soluble and are surrounded by water, so their surfaces are enriched in hydrophilic residues and their hydrophobic residues are buried in the interior. As a result, a hydropathy plot for a typical globular cytosolic protein shows mostly negative or modestly fluctuating values, without long, high positive peaks that correspond to membrane-spanning helices.
Although a cytosolic protein can contain some hydrophobic stretches, these are usually shorter and fewer, and do not form the repeating pattern seen for multi-pass membrane proteins. The presence of seven well-defined hydrophobic segments in the plot is inconsistent with a purely cytosolic, soluble protein, so option C cannot be correct.
Why option D is incorrect: “cannot say”
Hydropathy plots, while not perfect, are a standard and widely accepted tool for predicting transmembrane helices and the general class of a membrane protein from its primary sequence. When a plot shows several long, strong hydrophobic regions of appropriate length, it is usually sufficient to conclude that the protein is a multiple-pass integral membrane protein, even without additional experimental data.
In this question, the pattern of seven prominent hydrophobic peaks is very typical and provides enough information to classify the protein as a multiple-pass integral membrane protein. Therefore, saying “cannot say, I need more information” underestimates the predictive power of hydropathy analysis, and option D is incorrect.
Summary table of options
| Option | Protein type | Expected hydropathy plot features | Match with given plot? |
|---|---|---|---|
| A | Lipid-anchored membrane protein | Mostly hydrophilic with at most one short hydrophobic region for lipid attachment. | No; plot shows seven long hydrophobic peaks. |
| B | Multiple-pass integral membrane protein | Several long, strong positive peaks (∼18–25 residues) indicating multiple transmembrane helices. | Yes; seven distinct hydrophobic segments are visible. |
| C | Cytosolic protein | Overall hydrophilic profile; few or no long strong hydrophobic peaks; negative values dominate. | No; pattern is too hydrophobic and repetitive. |
| D | Cannot say, need more information | Would apply only if peaks were ambiguous, short, or insufficient to infer membrane topology. | No; data is sufficient to label as multipass. |