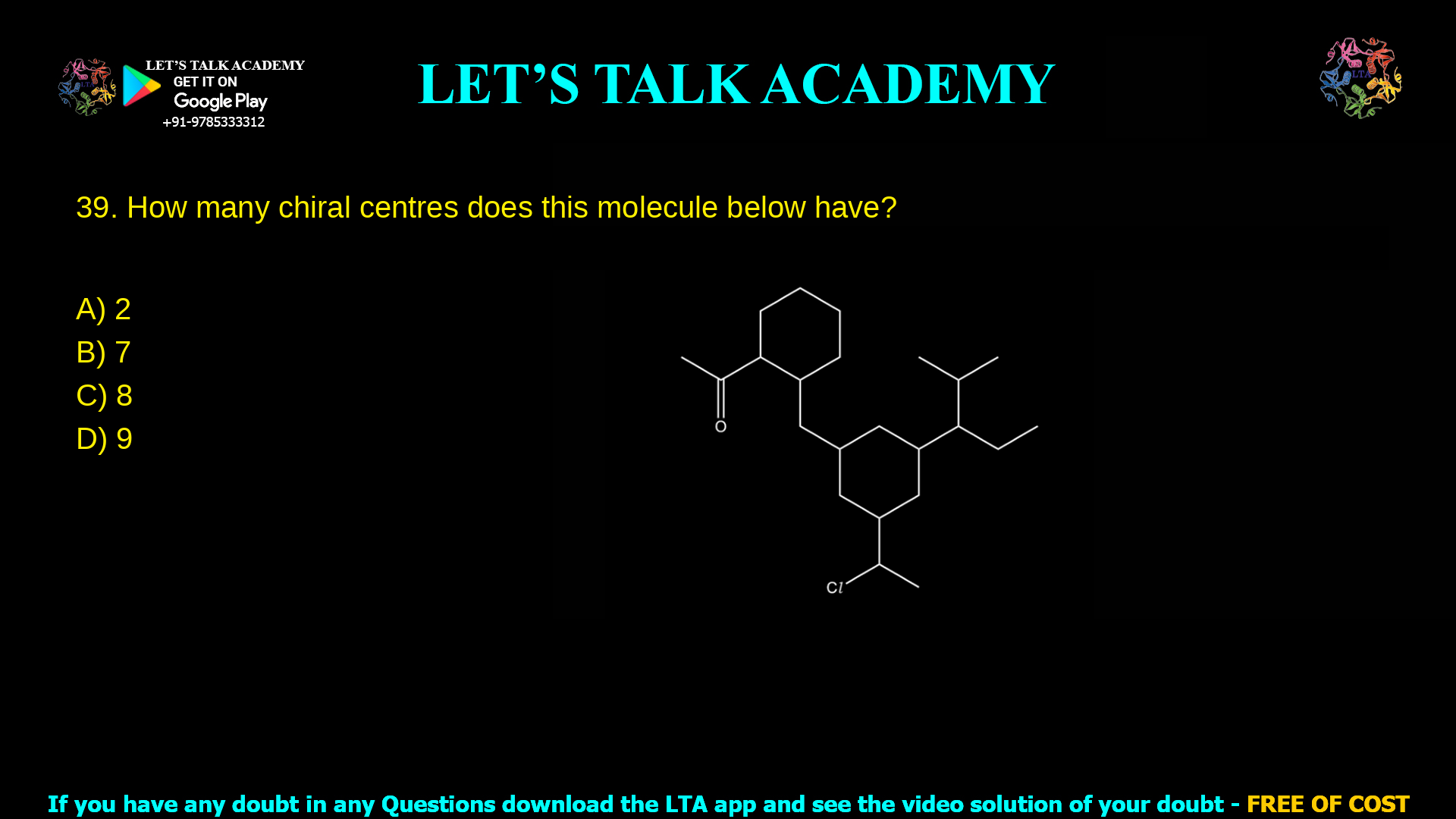
9. How many chiral centres does this molecule below have:
a. 2
b. 7
c. 8
d. 9
Introduction
Exam questions like “How many chiral centres does this molecule below have?” frequently appear in competitive chemistry and NET/JEE level exams, because they test both visualisation of 3‑D structures and conceptual understanding of chirality.
In this article, the structure from the image is analysed step by step to identify every stereogenic carbon, then each MCQ option (2, 7, 8, 9) is discussed to show why only 8 is correct.
Stepwise method to count chiral centres
-
A chiral centre (usually a tetrahedral carbon) is attached to four different groups, which makes its mirror image non‑superimposable.
-
In rings, the two paths around the ring can count as different groups if substitution breaks symmetry; if the two paths are identical (as in an unsubstituted or symmetrically substituted ring), that ring carbon is not chiral.
For the given molecule:
-
Ignore obviously achiral carbons
-
Carbonyl carbon of the ester is sp² (trigonal planar) and cannot be a chiral centre.
-
Any carbon with two identical substituents (e.g., CH₂, or a tertiary carbon with two identical alkyl groups) is not chiral.
-
-
Analyse the left cyclohexane ring
-
The ring‑junction carbon connecting the ester side chain and the ring is sp³ and attached to: the ester chain, the ring in one direction, the ring in the other direction (not equivalent because of substitution), and a hydrogen.
-
This carbon therefore has four different groups and is a chiral centre (1).
-
Within this left ring, two additional ring carbons each carry different substituents on an otherwise unsymmetrical ring (due to attachment to the central chain), giving two more sp³ carbons with four different groups (2 and 3).
-
-
Analyse the central cyclohexane ring
-
The carbon where the left ring chain enters, and the carbon where the right‑hand isopropyl‑like branch leaves, are both sp³ and see four different groups (two non‑equivalent ring paths, the side chain, and H). These two carbons are chiral centres 4 and 5.
-
A lower carbon in this ring bears a –CH(Cl)–CH₃ side chain, breaking any remaining symmetry of the ring and giving another chiral centre in the ring (6).
-
-
Analyse the –CH(Cl)–CH₃ side chain
-
The carbon directly attached to Cl is bonded to Cl, H, CH₃ and the rest of the carbon skeleton, which are four different groups. This gives chiral centre 7.
-
-
Analyse the right‑hand branched (isopropyl‑like) fragment
-
The junction carbon that connects this branch to the central ring is attached to three different carbon chains and a hydrogen (the two “methyl”‑looking ends are not equivalent because they sit on different parts of the skeleton), so this carbon is chiral centre 8.
-
Adding these stereocentres: 1 (left junction) + 2 (remaining in left ring) + 3 (in central ring) + 1 (Cl‑bearing side chain) + 1 (right branch junction) = 8 chiral centres.
Why each option is right or wrong
Option A: 2 chiral centres
A count of only 2 would occur if one considered just two obviously substituted ring carbons and ignored:
-
The ring‑junction carbons where two rings/branches meet.
-
The chlorine‑bearing carbon and asymmetric junction in the right‑side branch.
Because several additional sp³ carbons clearly have four different environments, 2 is far too low and therefore incorrect.
Option B: 7 chiral centres
Students sometimes obtain 7 by:
-
Correctly identifying all chiral centres in the two rings and the Cl‑bearing carbon,
-
But missing one of the junction carbons in the right‑hand branched fragment, assuming its two “alkyl groups” are identical when they are actually part of different chains.
Since careful path tracing shows that this junction sees four distinct groups, 7 undercounts the real total and is incorrect.
Option C: 8 chiral centres (Correct)
-
All tetrahedral carbons attached to four different groups (in both rings, the Cl‑bearing side chain, and the branched right‑hand fragment) are included.
-
No sp² carbon or symmetric CH₂/CH₃ carbon is mistakenly counted.
This consistent application of the chirality rule yields 8 stereogenic centres, so Option C is correct.
Option D: 9 chiral centres
To reach 9, at least one extra carbon must be incorrectly treated as chiral, typically:
-
A CH₂ inside a ring that actually has two identical ring paths, or
-
The carbonyl carbon of the ester, which is sp² and cannot be chiral.
Because such atoms do not have four distinct substituents, 9 overcounts the number of chiral centres and is therefore incorrect.
By following this systematic approach—eliminating sp² and symmetric carbons, then examining each sp³ carbon (especially ring junctions and side‑chain branching points)—questions of the type “how many chiral centres does this molecule have” can be solved quickly and accurately in exams.