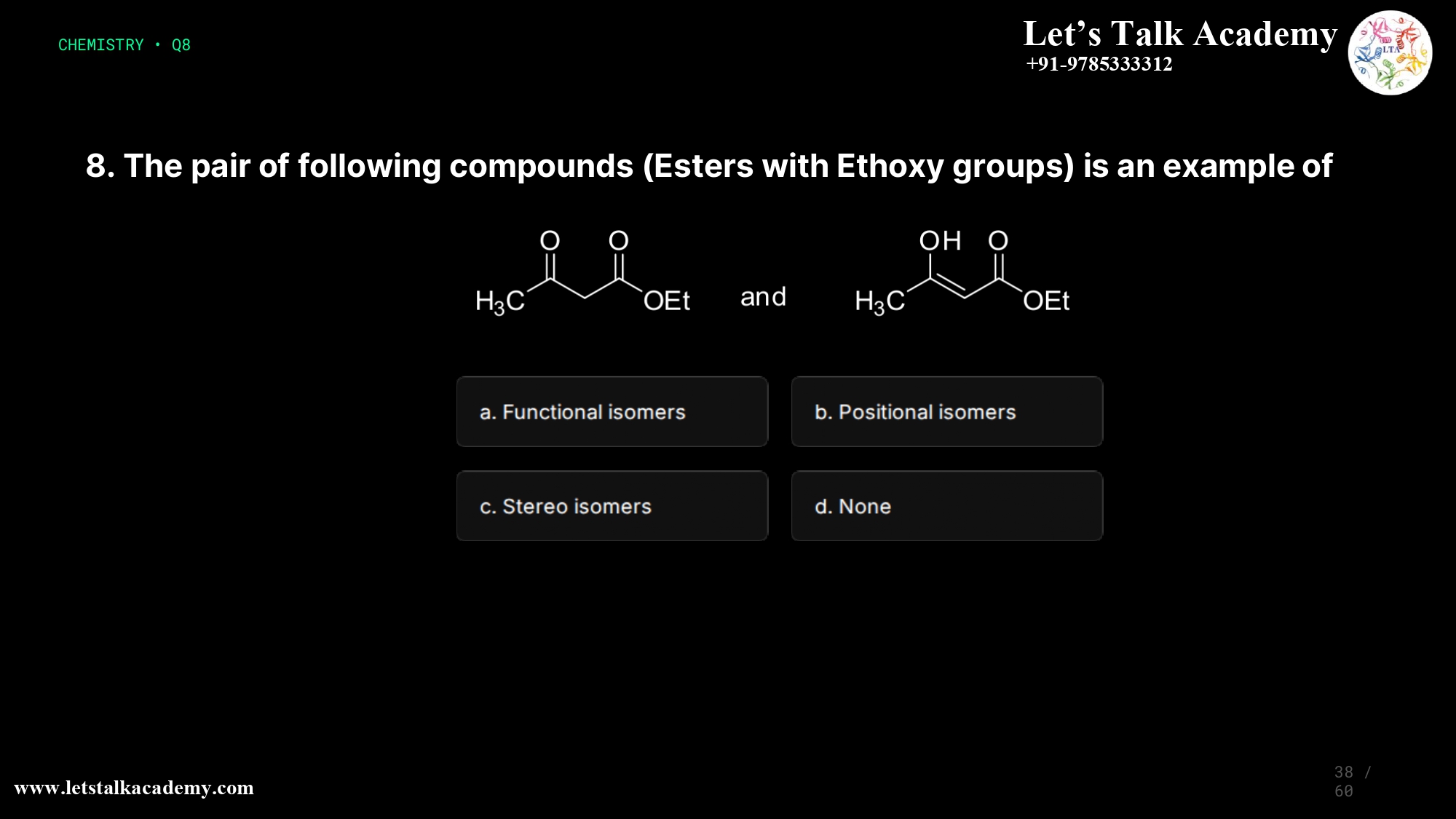
8. The pair of following compounds is an example of
a. Functional isomers
b. Positional isomers
c. Stereo isomers
d. None
Answer:
The two given compounds are functional isomers (option a). They have the same molecular formula but differ in functional group arrangement: one is a pure ester, while the other contains both an ester and an alcohol (hydroxy) group, so the overall functional grouping changes.
Understanding the Structures
From the image, the left compound is an ester with two carbonyl (C=O) groups and an ethoxy (OEt) group, while the right compound has one carbonyl (C=O), one hydroxyl (OH), and an ester group with OEt. Both share the same overall molecular formula but differ in the nature and connectivity of functional groups, fulfilling the definition of functional isomerism in organic chemistry.
Option a: Functional isomers (Correct)
Functional isomers have the same molecular formula but differ in functional group type or combination, leading to different chemical properties. In this pair, one structure behaves as a di-ester-type skeleton while the other behaves as a β‑hydroxy ester (alcohol + ester); the replacement of one C=O by an OH changes the functional grouping, so they are functional isomers.
Option b: Positional isomers (Incorrect)
Positional isomers share the same functional group and carbon skeleton, but the position of the functional group along the chain is different (for example, moving a nitro group from C‑2 to C‑3). In this question, the functional groups themselves are not the same in the two molecules (C=O vs OH), so they cannot be classified as positional isomers.
Option c: Stereo isomers (Incorrect)
Stereo isomers have the same molecular formula and connectivity but differ in spatial arrangement (e.g., cis–trans isomers, enantiomers, diastereomers). Here, the bonding pattern (connectivity) and functional group set differ; there is no chiral center or restricted rotation scenario generating stereoisomerism, so stereo isomerism is not applicable.
Option d: None (Incorrect)
Since the compounds clearly follow the definition of functional isomers, selecting “None” is unjustified. Recognizing them as functional isomers helps in quickly categorizing similar pairs in exam questions on organic isomerism.
Short SEO-Friendly Explanation
In exam-oriented organic chemistry, the pair of esters with ethoxy groups shown in the question is a classic example of functional isomerism in esters with ethoxy groups. One compound contains only carbonyl-based ester functionality, while the other converts one carbonyl to a hydroxyl group, producing a β‑hydroxy ester. This change in functional group type, while maintaining the same molecular formula, is the hallmark of functional isomers, not positional or stereoisomers.