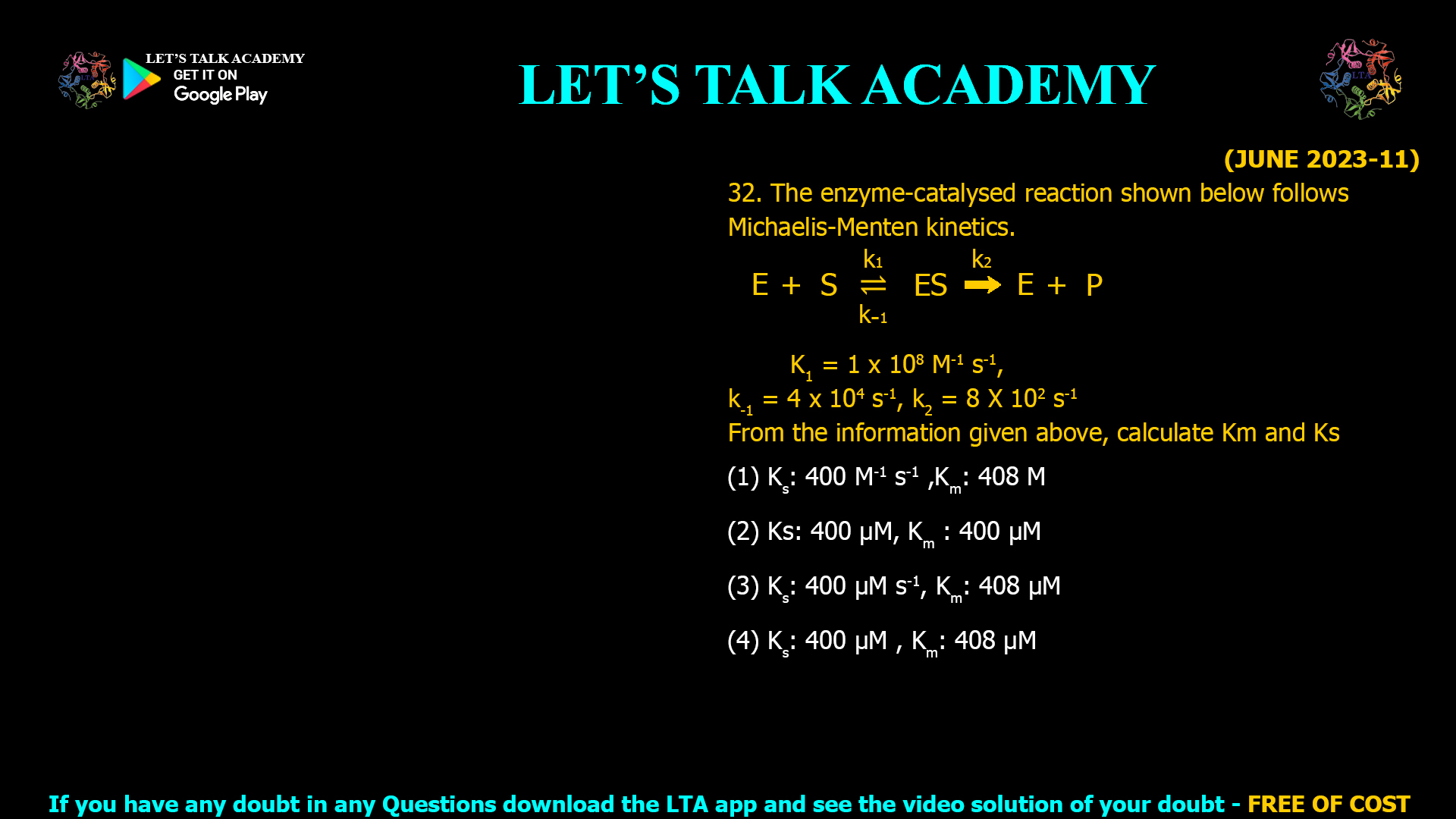
The correct answer is (4) Kₛ: 400 μM, Kₘ: 408 μM.
Introduction
Michaelis-Menten kinetics provides a foundation for understanding enzyme-catalyzed reactions. Two important constants—substrate dissociation constant (Ks) and Michaelis constant (Km)—help quantify substrate affinity and kinetic efficiency. This article walks through the calculation of Km and Ks for a given reaction using provided rate constants, culminating in an expertly reasoned answer, ideal for competitive exams and biochemistry training.
Stepwise Calculation
Given data:
-
k1=1×108 M−1 s−1 (association rate constant)
-
k−1=4×104 s−1 (dissociation rate constant)
-
k2=8×102 s−1 (catalytic rate constant/product formation)
1. Substrate Dissociation Constant (Ks)
Ks=k−1k1
Substitute values:
Ks=4×1041×108=4×10−4 M=400 μM
2. Michaelis Constant (Km)
Km=k−1+k2k1
Substitute values:
Km=4×104+8×1021×108=4.08×1041×108=4.08×10−4 M=408 μM
Interpretation
-
Ks (400 μM) reflects the enzyme’s basic affinity for the substrate, independent of turnover.
-
Km (408 μM) incorporates both substrate binding and catalytic conversion, always slightly higher than Ks if product formation (k2) is significant.
-
The tiny difference between Km and Ks reflects rapid substrate binding and reasonably efficient product formation.
Significance in Biochemistry
-
Calculating both constants helps in understanding not only how an enzyme binds a substrate but also how efficiently it turns a bound substrate into product.
-
Km is widely used to compare enzyme efficiency and substrate preference, while Ks focuses solely on binding.
-
In experimental design, knowing specific values allows optimization of substrate concentrations to study enzyme kinetics, inhibition, and efficiency.
Summary Table
| Constant | Formula | Value | Unit |
|---|---|---|---|
| Ks | k−1/k1 | 400 | μM |
| Km | (k−1+k2)/k1 | 408 | μM |
Conclusion
For the enzyme-catalyzed reaction in question, the calculated values from rate constants are Ks: 400 μM and Km: 408 μM, matching option (4). This demonstrates both substrate binding and catalytic turnover, providing rich insight into enzyme kinetics essential for advanced biochemistry learning and research.



17 Comments
Aakansha sharma Sharma
September 12, 2025We know Km=kb1+kcat/kf1 but here km=k-1+k2/k1 so k-1=4×10(4)s-1 +8×10(2)s-1/1×10(8)M-1s-1
=4000s-1+800s-1/1×100000000M-1s-1
=40800s-1/100000000M-s-1
408s-1/1000000M-1s-1
= 408 × 1000000s-1/1000000Ms-1
=408M=Km
Ks here is dissociation constants so Ks=kb1/kf1 here it is K-1/K1 so
4×10(4)s-1/1×10(8) M-1S-1
40000×1000000s-1/100000000M-1s-2
40000/100M
400M=Ks
So answer isKm=408M,Ks=400M
Varsha Tatla
September 13, 2025Solved
Khushi Vaishnav
September 13, 2025Kₛ: 400 μM,
Kₘ: 408 μM.
Pooja
September 14, 2025Option 4 is correct
Kanica Sunwalka
September 14, 2025Km= Kb1+ Kcat / Kf1
here Km = K-1 + K2 / K1
so on calculating we get 408 x 10^6 = 408uM
Dissociation constant , Ks = Kb1/ Kf1
here Ks= K-1 / k1
on calculating we get 4 x 10^ -4
now to convert it into uM -> we multiply it by 10 ^6
Ks = 400 uM
Km = 408 uM
Ks = 400 uM
Kajal
September 14, 2025Ks=400uM
Km=408uM
Are the values
Rishita
September 14, 2025Kₛ: 400 μM, Kₘ: 408 μM.
Neha Yadav
September 14, 2025Ks = 400 um
Km = 408 um
Tanvi Panwar
September 14, 2025Ks=Kb1/Kf1=400 um
Km=Kb1+K2/K1 = 408 um.
Manisha choudhary
September 14, 20254th answer is right
Ayush Dubey
September 15, 2025Kₛ: 400 μM, Kₘ: 408 μM.
Khushi Agarwal
September 15, 2025The correct answer is (4)
Kₛ: 400 μM, Kₘ: 408 μM
Pallavi Ghangas
September 15, 20254
Kirti Agarwal
September 15, 2025Opt 4
Anjana sharma
September 16, 2025Kₛ: 400 μM, Kₘ: 408 μM.
Palak Sharma
September 16, 2025Kₛ: 400 μM, Kₘ: 408 μM.
Arushi Saini
September 16, 2025The correct answer is (4)
Kₛ: 400 μM, Kₘ: 408 μM