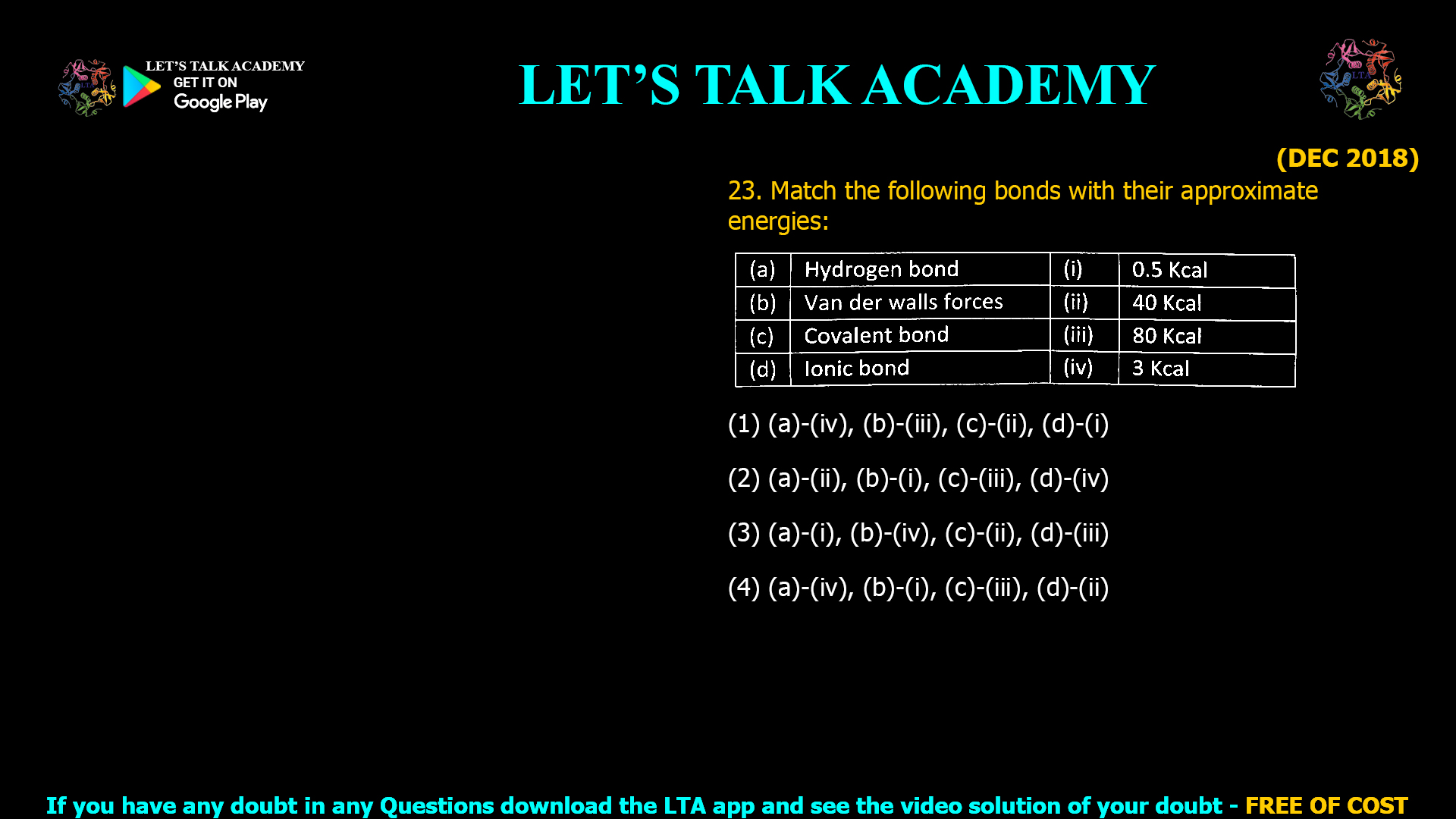
- Match the following bonds with their approximate energies:

(1) (a)-(iv), (b)-(iii), (c)-(ii), (d)-(i)
(2) (a)-(ii), (b)-(i), (c)-(iii), (d)-(iv)
(3) (a)-(i), (b)-(iv), (c)-(ii), (d)-(iii)
(4) (a)-(iv), (b)-(i), (c)-(iii), (d)-(ii)Introduction to Molecular Interactions
Molecular interactions define the stability, structure, and function of biomolecules such as proteins, DNA, and cell membranes. Hydrogen bonds, van der Waals forces, hydrophobic interactions, and electrostatic interactions are some of the most significant. Each of these varies in their strengths and energy values, impacting biological processes.
Comparison of Various Types of Molecular Interactions
1. Hydrogen Bonds
Strength: Stronger than van der Waals forces but weaker than a covalent bond.Energy: 20–40 kJ/mol.
Formation: Electrostatic attraction between a hydrogen atom bonded to O, N, or F and a second electronegative atom.
Importance: Critical in protein folding, DNA base pairing (A-T, G-C), and enzyme-substrate interaction.
2. Van der Waals Forces
Strength: Least strong intermolecular force.
Energy: 0.4–4 kJ/mol.
Formation: Slight electron oscillations form dipoles, resulting in weak attractions.
Importance: Contributes to protein structure, molecular recognition, and material properties.
3. Hydrophobic Interactions
Strength: Stronger than hydrogen bonds but essential for molecule stability.Energy: Not explicitly defined but greatly contributing to biomolecule stability.
Formation: Hydrophobic molecules come together in water to limit entropy loss.
Importance: Important for folding of proteins, formation of membranes, and interaction between lipids.
4. Electrostatic Interactions ✅
Strength: Charge magnitude-dependent, distance-dependent, and environmentally dependent.
Energy: Weak or strong, dependent on ionic strength.
Formation: Attractions or repulsions between atoms or molecules having charge.
Importance: Essential for salt bridge formation, protein stability, and nucleic acid interactions.
Assigning Bonds to Their Rough Approximate Energies

Correct Answer: (1) (a)-(iv), (b)-(iii), (c)-(ii), (d)-(i) ✅
Significance of KnowingMolecular Interactions1. Protein Stability & Folding
Hydrophobic interactions and hydrogen bonds stabilize protein structure.2. DNA Base Pairing & Genetic Information
Hydrogen bonding provides correct base pairing during DNA replication.3. Drug Design & Molecular Recognition
Knowledge of binding energies assists in designing drugs that are effective.Conclusion
Every molecular interaction has a singular and vital function in biological systems. Hydrogen bonds, van der Waals forces, hydrophobic interactions, and electrostatic interactions differ in energy and strength but collectively serve to stabilize proteins, DNA, and cell membranes.



10 Comments
Prami Masih
March 31, 2025Done sir ji
Pallavi gautam
March 31, 2025Option 4 is correct
Arushi
March 31, 2025👍☑️
Arushi
March 31, 2025👍✔️
Parul
March 31, 2025Done sir.
Mohit Akhand
March 31, 2025Option (4) correct
Manisha
April 1, 2025Correct option is 4 done ✅✅
Manisha
April 1, 2025Correct option is 4
done sir ✅✅
Lokesh Kumawat
April 1, 2025Done .I think 4 is correct? ?
Parul
April 7, 2025Option 4 is correct