21. If van der Waals interaction is described by the following relation.
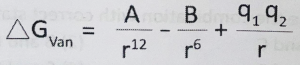
Where ΔGvan is the free energy of the van der Waals interaction, A and B are constants, r is the distance between two nonbonded atoms 1 and 2 and q1 and q2 are partial charges on the dipoles 1 and 2. In this relation, the parameter A describes
(1) electron shell attraction
(2) electron shell repulsion
(3) dipole-dipole attraction
(4) dipole-dipole repulsion
Introduction to Van der Waals Interactions
Van der Waals forces are weak intermolecular forces that are important in the stability of molecules, protein folding, and biomolecular interactions. These forces originate from temporary dipoles, permanent dipoles, and induced dipole effects between nonbonded atoms.
The van der Waals interaction is usually represented by the equation:
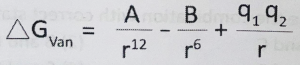
where:
ΔGvan = Free energy of van der Waals interaction
A and B = Constants
r = Distance between two nonbonded atoms
q₁ and q₂ = Partial charges on dipoles
What Does Parameter A Represent?
The equation has two competing forces:
Attractive term (−B/r⁶) – Represents London dispersion forces (induced dipole attraction).
Repulsive term (A/r¹²) – Represents electron shell repulsion due to the Pauli exclusion principle.
Correct Answer: (2) Electron Shell Repulsion
A/r¹² accounts for electron shell repulsion, which keeps atoms from imploding into one another.
Repulsion occurs since electrons in intersecting atomic orbitals are subjected to strong repulsive forces.
It provides stability to molecules by opposing attractive forces.
Why Other Choices Are Incorrect?
1. Electron Shell Attraction ❌
Attraction is due to London dispersion forces, symbolized by B/r⁶, not A/r¹².
2. Dipole-Dipole Attraction ❌
Dipole interactions take place in polar molecules, but the equation of van der Waals works with all molecules, including nonpolar ones.
3. Dipole-Dipole Repulsion ❌
There is dipole repulsion but is not accounted for by parameter A. However, A is used to represent electron cloud repulsion.
Role of Van der Waals Forces in Biochemistry
1. Protein Folding & Stability
Proteins are stabilized by van der Waals forces.
2. Membrane Formation
They stabilize cell membranes and lipid bilayers.
3. Drug Design & Molecular Recognition
These interactions are what affect ligand binding to enzymes and receptors.
Conclusion
In the van der Waals equation, parameter A is due to the Pauli exclusion principle and represents electron shell repulsion. This force keeps atoms from collapsing into one another and counters attractive forces to ensure that molecules are stable.




7 Comments
Beena Meena
March 30, 2025Best explanation sir
Beena Meena
March 30, 2025Best explanation 👍
Prami Masih
March 31, 2025Okay sir ji
Arushi
April 1, 2025👍👍✔️
Lokesh kumawat
April 1, 2025…..
Ujjwal
April 2, 2025👍👍
Parul
April 4, 2025Done with the help of explanation.