-
The interaction energy between two opposite charges separated by 3Å in vacuum is -500 kJmol-1. The interaction energy between these two charges in water will be closest to
(1) -1500 kJmol-1
(2) -166 kJmol-1
(3) -55 kJmol-1
(4) -6 kJmol-1
Interaction Energy Between Opposite Charges in Water vs. Vacuum
Understanding Electrostatic Interaction Energy
Electrostatic interaction energy is a basic concept of chemistry and physics that explains the interaction between charged particles. The energy between two opposite charges is a function of the medium in which they exist. This is an important concept in understanding molecular interactions, chemical bonding, and solvation effects.
Given Problem:
Calculation of Interaction Energy
We have given:
Charge Separation: 3Å (0.3 nm)
Interaction Energy in Vacuum: -500 kJ/mol
Mediums to Compare: Vacuum
and Water
The interaction energy
according to Coulomb‘s law is

where ε is the permittivity of the medium. The dielectric constant (relative permittivity) of vacuum is 1, while for water, it is approximately 78.5 at room temperature.
Effect of Water on Interaction Energy
Since water has a high dielectric constant, it reduces the interaction energy significantly. The interaction energy in water is given by:
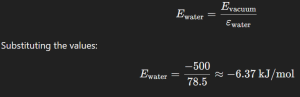
Therefore, the correct answer is (4) -6 kJ/mol.
Importance of Dielectric Constant in Chemistry
1. Role in Solvation and Chemical Reactions
The high dielectric constant of water renders it a good solvent for ionic substances. It reduces electrostatic forces, enabling salts and polar molecules to dissolve readily.
2. Impact on Biological Systems
In biological systems, the dielectric constant affects protein folding, enzyme function, and molecular interactions. Most biochemical processes depend on the capacity of water to regulate charge interactions.
Conclusion
The energy of interaction between charges of opposite signs is much decreased in water because of its high dielectric constant. This concept is a cornerstone of chemistry, physics, and biology to describe solvation, ion dissociation, and molecular interactions. The knowledge of electrostatic forces is useful for predicting reaction behaviors and creating more efficient chemical and biological systems.




8 Comments
Suman bhakar
March 30, 2025👍👍
Prami Masih
April 1, 2025👍👍👍👍
Manisha
April 1, 2025Fair/ dielectric of medium ✅✅
Manisha
April 1, 2025Fair
/ dielectric of medium ✅✅
Done ✅
Akshay mahawar
April 1, 2025Done 👍
Pallavi gautam
April 2, 2025Done
Arushi
April 3, 2025👍✔️
Parul
April 5, 2025Done sir. With the help of explanation.