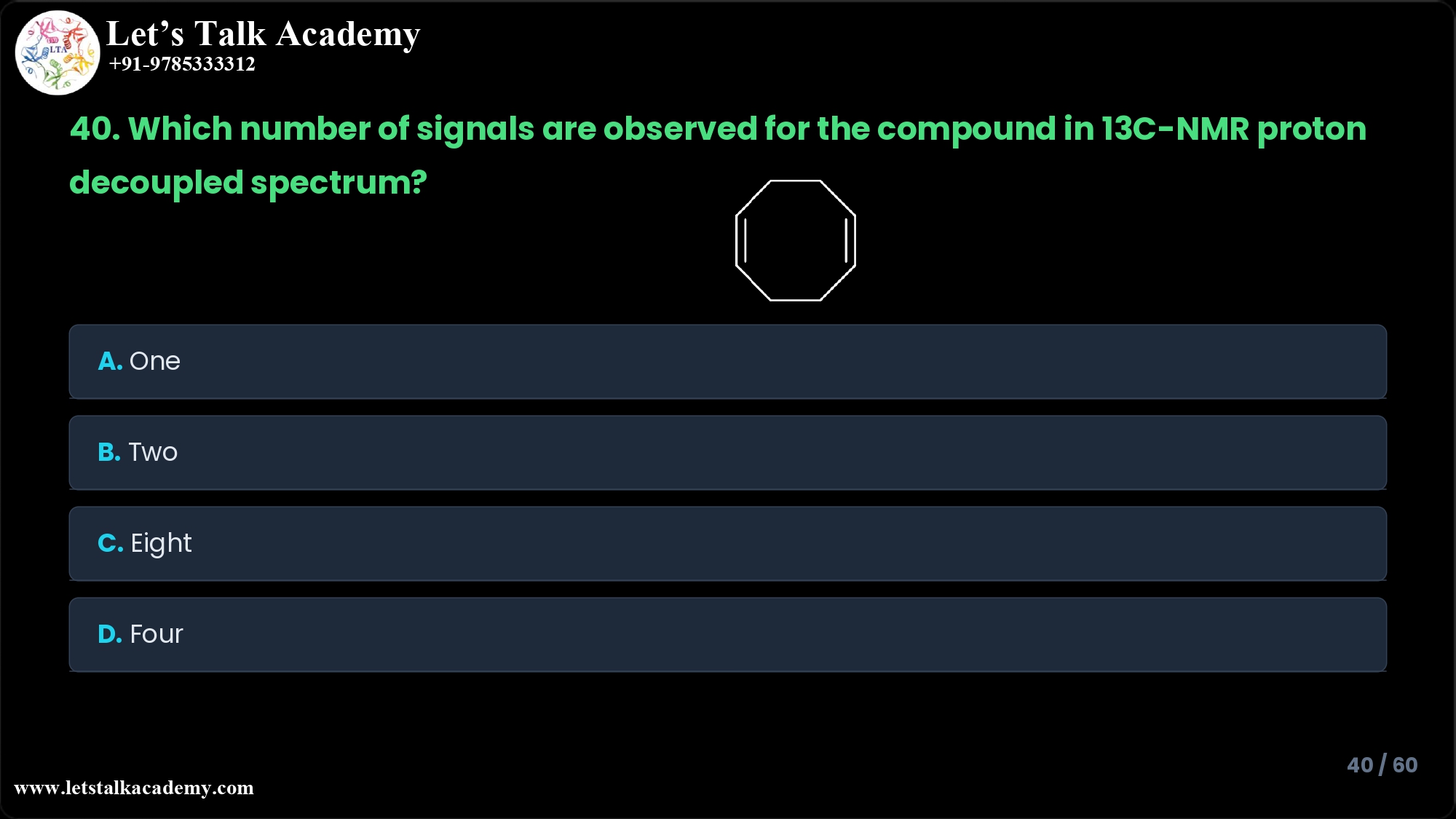
40. Which number of signals are observed for the following compound in 13C-NMR
proton decoupled spectrum?
A. One
B. Two
C. Eight
D. Four
The correct answer is A. One signal in the proton‑decoupled 13C‑NMR spectrum.
Introduction
In proton‑decoupled 13C‑NMR, each set of chemically and magnetically equivalent carbons appears as one signal, regardless of how many identical carbons are present. For highly symmetric rings like cyclooctane in its most stable conformation, symmetry operations make all carbon atoms equivalent, collapsing the spectrum to a single 13C resonance. This concept is frequently tested in spectroscopy MCQs in chemistry entrance and competitive exams.
Structure and Symmetry Analysis
The compound shown is an eight‑membered saturated ring (cyclooctane) with no heteroatoms or substituents.
-
All ring positions are CH2 groups attached only to neighboring carbons (no branching, no double bonds).
-
In its preferred conformations, cyclooctane has high symmetry, so every carbon can be superimposed on any other carbon through symmetry operations (rotations and reflections).
Because of this complete equivalence, all eight carbons experience the same electronic environment and therefore resonate at the same chemical shift in a proton‑decoupled 13C‑NMR spectrum, giving one single signal.
Option‑wise Explanation
Option A: One
-
In proton‑decoupled 13C‑NMR, the number of signals equals the number of sets of equivalent carbons, not the total number of carbons.
-
For cyclooctane, symmetry makes all eight carbons equivalent, so there is only one set of carbons and hence one 13C signal.
-
Therefore, Option A (One) is correct.
Option B: Two
-
Two signals would mean the ring carbons are divided into two distinct environments (e.g., alternating types or axial/equatorial carbons that are not interconverted rapidly on the NMR timescale).
-
In cyclooctane, rapid conformational averaging and overall symmetry make all carbons equivalent; there is no persistent pair of distinct carbon types.
-
Hence, Option B is incorrect because the structure does not produce two nonequivalent carbon sets.
Option C: Eight
-
Eight signals would correspond to eight completely nonequivalent carbons, as in an unsymmetrical substituted ring.
-
The given molecule is unsubstituted cyclooctane with full rotational symmetry; individual ring positions are indistinguishable.
-
Thus, Option C is wrong; counting raw atoms (8 carbons) without considering symmetry is a common trap.
Option D: Four
-
Four signals would suggest four pairs of equivalent carbons, as seen in some less symmetric cyclic systems or substituted rings where opposite carbons are related by symmetry while neighbors are not.
-
Cyclooctane’s symmetry is higher than this; no such subdivision into four nonequivalent pairs occurs in the time‑averaged NMR.
-
Therefore, Option D is also incorrect.
Final Answer: The number of signals observed in the proton‑decoupled 13C‑NMR spectrum of this cyclooctane is one (Option A).