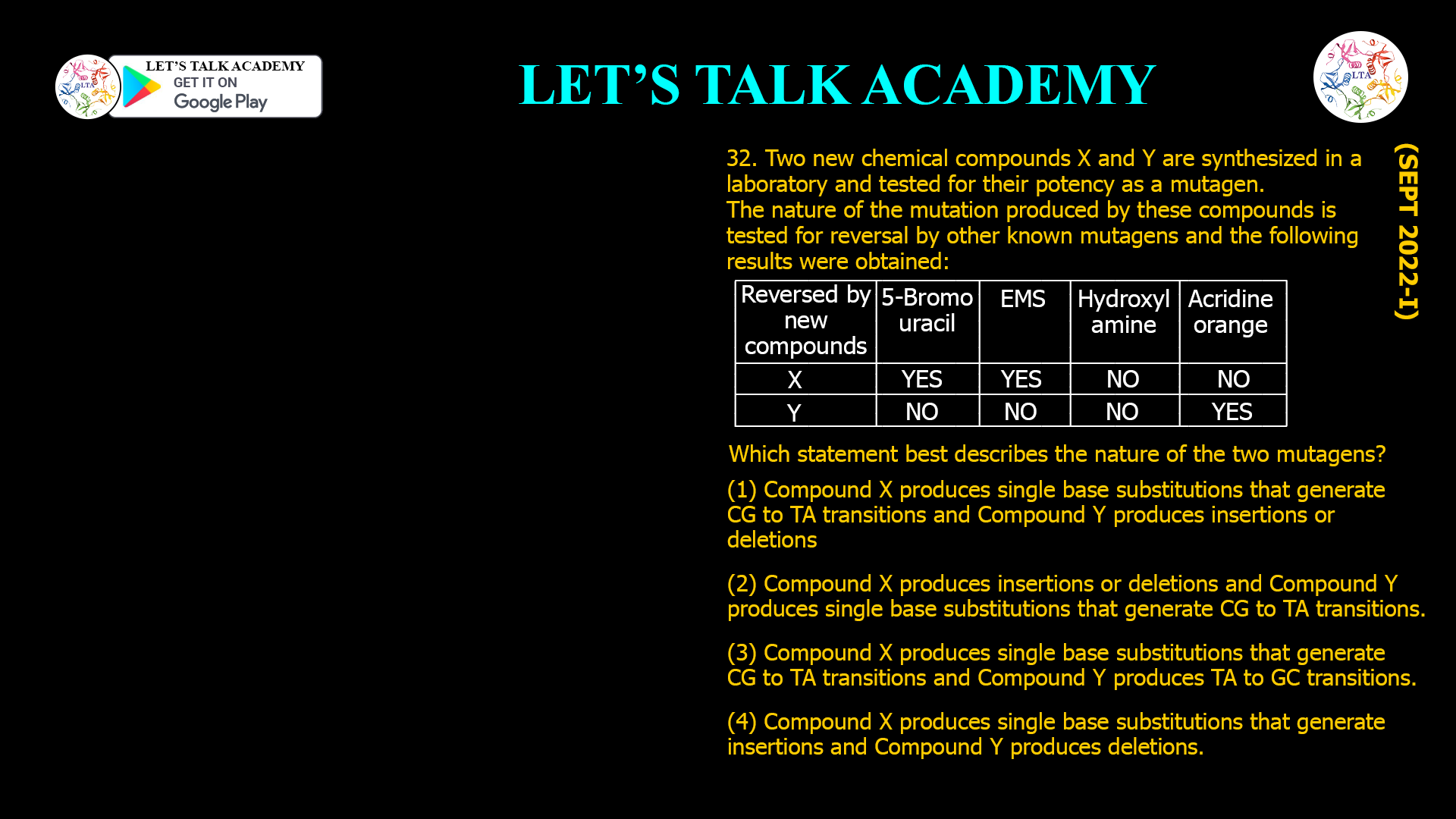
- Two new chemical compounds X and Y are synthesized in a laboratory and tested for their potency as a mutagen, The nature of the mutation produced by these compounds is tested for reversal by other known mutagens and the following results were obtained:
| Reversed by new compounds | 5-Bromo uracil | EMS | Hydroxyl amine | Acridine orange |
| X | Yes | Yes | No | No |
| Y | No | No | No | Yes |
Which statement best describes the nature of the two mutagens?
(1) Compound X produces single base substitutions that generate CG to TA transitions and Compound Y produces insertions or deletions
(2) Compound X produces insertions or deletions and Compound Y produces single base substitutions that generate CG to TA transitions.
(3) Compound X produces single base substitutions that generate CG to TA transitions and Compound Y produces TA to GC transitions.
(4) Compound X produces single base substitutions that generate insertions and Compound Y produces deletions.
Compound X causes single base substitutions (CG to TA transitions), and compound Y causes insertions or deletions (frameshift mutations).
Question summary
Two new mutagens, X and Y, are analyzed by testing whether their induced mutations can be reversed by four standard mutagens: 5‑bromouracil, EMS, hydroxylamine, and acridine orange.
Mutations made by X are reversed by 5‑bromouracil and EMS but not by hydroxylamine or acridine orange, whereas mutations made by Y are reversed only by acridine orange.
Key concepts about standard mutagens
5‑Bromouracil is a base analog of thymine that induces transition mutations, converting A:T to G:C or vice versa via base substitution.
EMS (ethyl methanesulfonate) and hydroxylamine are base‑altering mutagens that mainly cause G:C to A:T transitions (CG to TA) by modifying guanine or cytosine.
Acridine orange is an intercalating agent that inserts itself between base pairs and typically causes or reverses frameshift mutations, i.e., single‑base insertions or deletions.
Deducing the nature of mutagens X and Y
Because X‑induced mutations are reversed by 5‑bromouracil and EMS, both transition‑inducing mutagens, X must also cause base‑substitution (point) mutations rather than frameshifts.
The pattern, especially reversal by EMS and not by hydroxylamine, is diagnostic of transitions of the type G:C to A:T (CG to TA transitions).
Mutations caused by Y are reversed only by acridine orange, which is specific for frameshift (insertion/deletion) mutations, so Y must cause insertions or deletions of bases.
Evaluation of each option
-
Option (1): “Compound X produces single base substitutions that generate CG to TA transitions and Compound Y produces insertions or deletions.”
This matches the conclusion: X causes CG→TA transitions (a type of base substitution), and Y causes frameshift mutations (insertions/deletions).
Hence, Option (1) is correct. -
Option (2): “Compound X produces insertions or deletions and Compound Y produces single base substitutions that generate CG to TA transitions.”
This contradicts the reversal pattern because X is reversed by transition mutagens and not by the frameshift mutagen, while Y is reversed only by the frameshift mutagen.
Therefore, Option (2) is incorrect. -
Option (3): “Compound X produces single base substitutions that generate CG to TA transitions and Compound Y produces TA to GC transitions.”
The description of X is fine, but Y is reversed only by acridine orange, which does not typically reverse simple base‑pair transitions; it acts on frameshift mutations.
Thus, Option (3) is incorrect. -
Option (4): “Compound X produces single base substitutions that generate insertions and Compound Y produces deletions.”
Insertions and deletions are frameshift events, not single‑base substitutions, and X is clearly associated with transition mutagens rather than frameshift mutagens.
Also, the table does not distinguish between insertions and deletions for Y, only that acridine orange can reverse its mutations, so this option misinterprets the data.
Hence, Option (4) is incorrect.
Correct answer: Option (1).
Introduction (SEO‑optimized)
Mutagenic analysis of new chemical compounds often relies on reversal tests using standard mutagens such as 5‑bromouracil, EMS, hydroxylamine, and acridine orange. In this CSIR‑NET style question, compounds X and Y are characterized by which of these known mutagens can reverse the mutations they induce, allowing precise identification of CG to TA transition mutations and frameshift insertions or deletions.
This detailed solution explains how each standard mutagen works, interprets the reversal pattern to determine the mutagenic nature of compounds X and Y, and analyses all four answer options one by one for conceptual clarity.