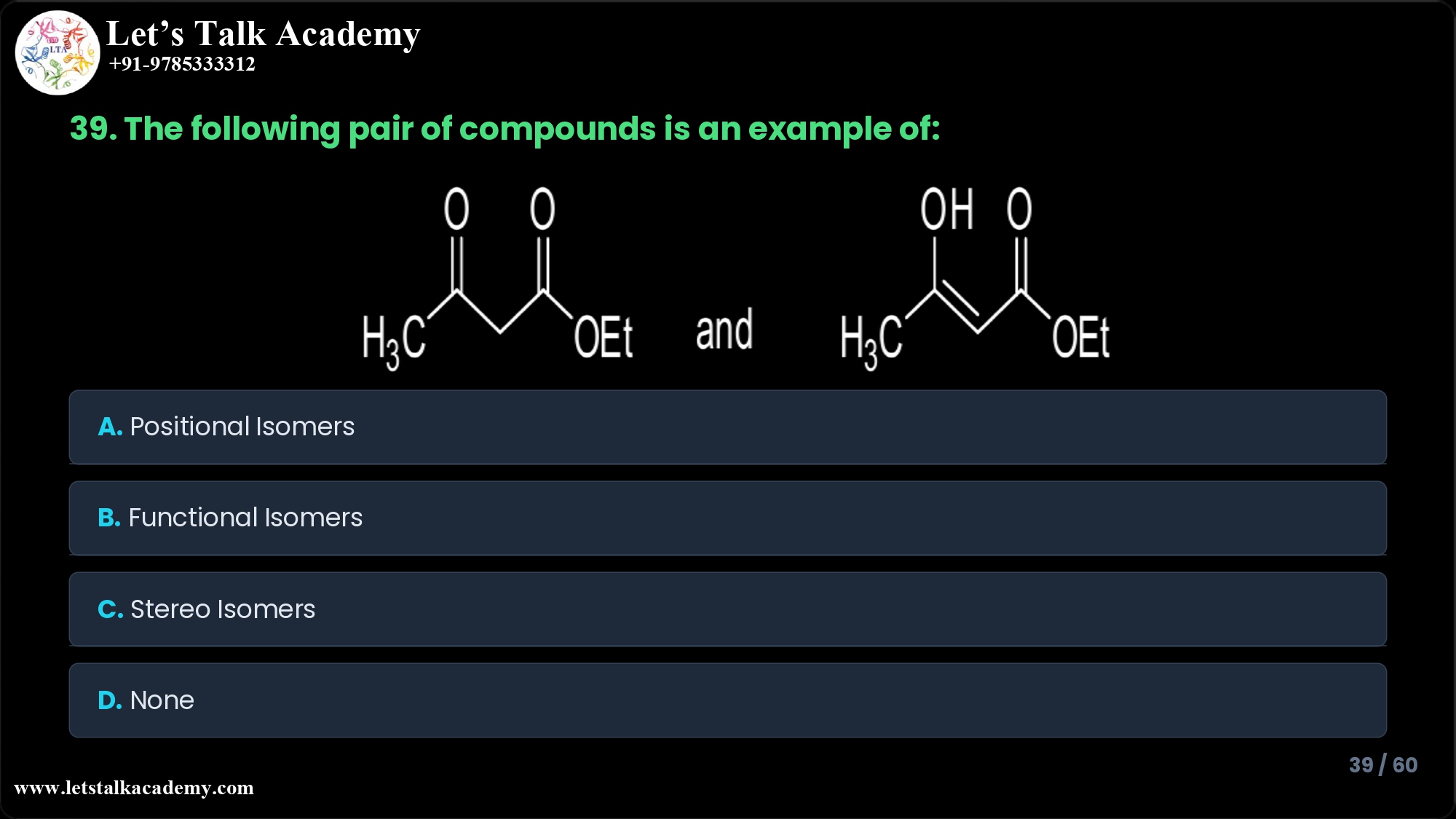
39. The following pair of compounds is an example of
A. Positional Isomers
B. Functional Isomers
C. Stereo Isomers
D. None
The given pair of compounds represents keto–enol tautomers, which are a special case of structural (constitutional) isomerism and are often treated as functional isomers because the functional group changes from a carbonyl (keto) to an enol (alkene + alcohol). Therefore, among the given options, the best correct answer is B. Functional isomers.
Introduction (SEO-optimized)
In organic chemistry, questions on isomerism often test the subtle differences between positional isomers, functional isomers, and stereoisomers, especially when keto–enol tautomerism is involved. The given pair of compounds (a β‑keto ester and its corresponding enol form) provides a classic example where recognizing the change in functional group is essential for marking the correct option in competitive exams.
Understanding the pair of compounds
The first structure is a β‑keto ester: it contains a carbonyl group (C=O) adjacent to a methylene (–CH2–) and an ester group (–COOEt).
The second structure shows an –OH attached to a carbon that is part of a C=C double bond, making it an enol form of the same β‑keto ester skeleton.
In keto–enol tautomerism, a proton shifts from the α‑carbon to the carbonyl oxygen and the double bond rearranges, converting the keto form into an enol form. The two forms are constitutional isomers (atoms connected differently) that rapidly interconvert and are called tautomers.
Why option B is correct: Functional isomers
-
Definition: Functional isomers have the same molecular formula but differ in functional group, such as alcohol vs ether, or ketone vs enol.
-
In the first compound, the key functional unit at that position is a ketone carbonyl (C=O), whereas in the second compound it has become an enol (alkene + alcohol, C=C–OH).
-
Because the molecular formula is unchanged but the functional group has changed from keto to enol, these two structures qualify as functional isomers; keto–enol tautomerism is often treated as a special case of functional isomerism / tautomeric isomerism in exam syllabi.
Therefore, Option B. Functional isomers is the best answer.
Why option A is incorrect: Not positional isomers
-
Position isomerism arises when the same functional group or multiple bond is attached at different positions on the same carbon skeleton (for example, moving a –Br or a double bond along the chain).
-
In the given pair, the carbon skeleton is the same, but the functional group itself has changed (keto vs enol) rather than merely its position.
-
Because there is a functional group change and not just a positional shift, the structures cannot be classified as positional isomers.
So, Option A is not correct here.
Why option C is incorrect: Not stereoisomers
-
Stereoisomers have the same connectivity of atoms but differ in spatial arrangement, such as cis–trans isomers or enantiomers.
-
In this question, the connectivity changes: a C=O in the keto form becomes C–O–H plus a C=C in the enol form, so bonds are arranged differently, meaning they are not stereoisomers but structural isomers.
Thus, Option C is ruled out.
Why option D is incorrect: Not “none”
-
The pair clearly shows the keto and enol tautomers of a β‑keto ester, which are recognized as tautomeric structural isomers and commonly grouped under functional isomerism at CSIR/NET/JEE level.
-
Since they do fit a standard, named category (functional / tautomeric isomers), the answer cannot be “none”.
Therefore, Option D is also incorrect.
Summary table: how the options differ
| Option | Type of isomerism | Key feature | Does it match this pair? | Reason |
|---|---|---|---|---|
| A | Positional isomerism | Same functional group, different position on chain | No | Functional group changes from keto to enol, not just position. |
| B | Functional isomerism | Same formula, different functional groups | Yes | Keto (C=O) vs enol (C=C–OH) forms of same β‑keto ester. |
| C | Stereoisomerism | Same connectivity, different 3D arrangement | No | Connectivity itself changes during tautomerization. |
| D | None of these | No standard isomeric relationship | No | They are classic keto–enol tautomeric/functional isomers. |
Hence, the correct choice is B. Functional isomers, specifically a classic example of keto–enol tautomerism frequently tested in organic chemistry exams.