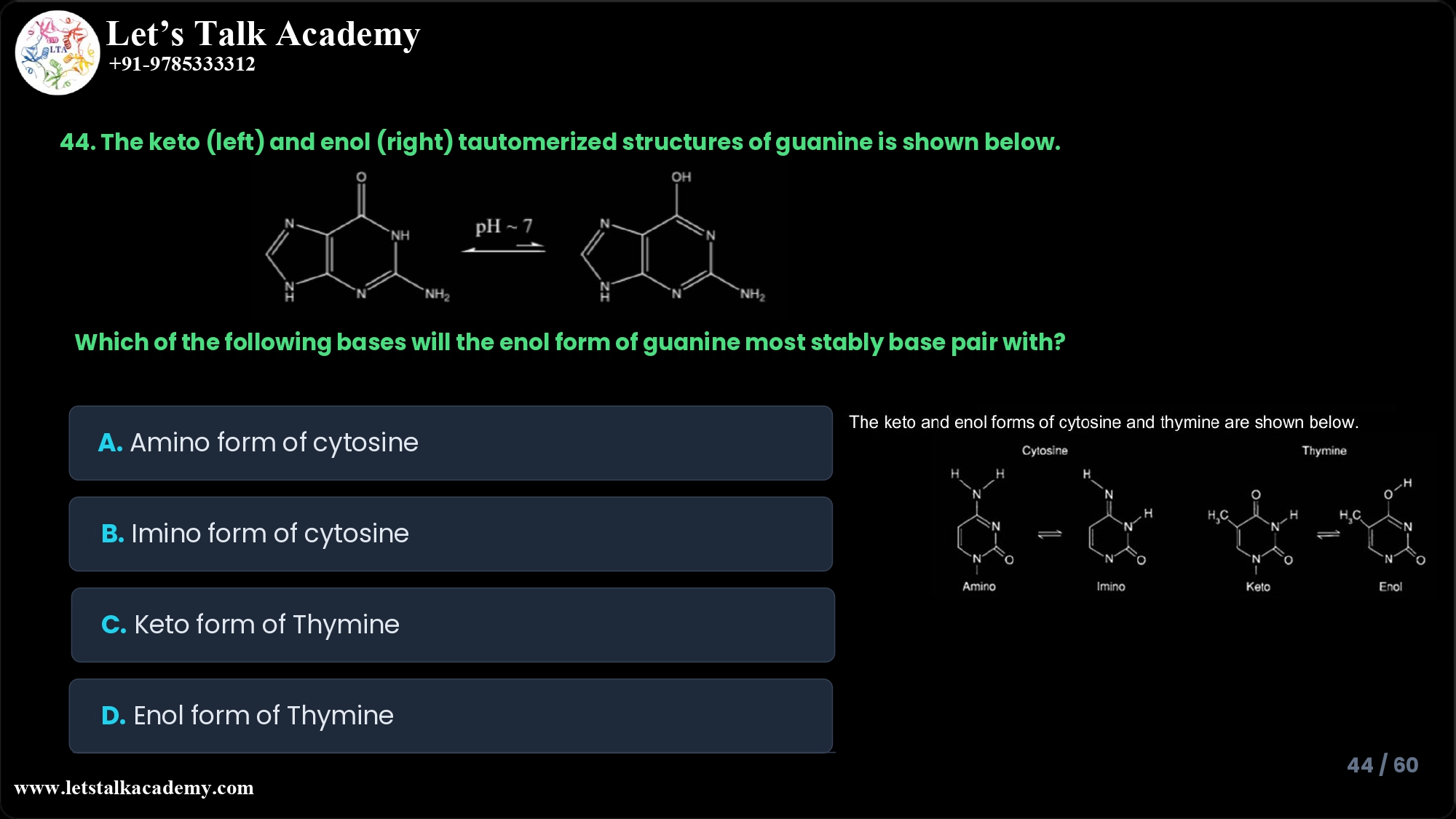
44. The keto (left) and enol (right) tautomerized structures of guanine is shown
below.
The keto and enol forms of cytosine and thymine are shown below.
Which of the following bases will the enol form of guanine most stably base pair with
A. Amino form of cytosine
B. Imino form of cytosine
C. Keto form of Thymine
D. Enol form of Thymine
The enol tautomer of guanine forms the most stable base pair with the enol form of thymine, so the correct option is D. Enol form of thymine.
Introduction
In DNA, correct base pairing depends on precise arrangements of hydrogen-bond donors and acceptors in the common keto and amino tautomers of the bases. When a base shifts into a rare enol or imino tautomer, its hydrogen-bonding pattern changes and it can mispair with an unusual partner, generating mutations and exam questions like this one.
The question:
“The keto (left) and enol (right) tautomerized structures of guanine are shown. Which of the following bases will the enol form of guanine most stably base pair with?
A. Amino form of cytosine
B. Imino form of cytosine
C. Keto form of thymine
D. Enol form of thymine”
Concept: Tautomers and base pairing
In standard Watson–Crick pairing, guanine is in the keto form and cytosine in the amino form, forming three hydrogen bonds with a specific donor–acceptor pattern. Thymine and adenine similarly pair when thymine is in the keto form and adenine in the amino form, giving two hydrogen bonds.
Keto–enol and amino–imino tautomerism shifts protons and changes which atoms are hydrogen-bond donors and acceptors on each base. When guanine adopts the enol tautomer, its pattern no longer fits cytosine’s normal amino form and instead can complement rare tautomers such as the enol form of thymine, leading to non‑canonical but relatively stable base pairs.
Why option D is correct (enol thymine)
Computational and theoretical studies show that the enol tautomer of guanine (enol‑G) can form favorable hydrogen bonding with thymine (T) when thymine is also in its enol tautomer (enol‑T). In this pairing, the hydroxyl and ring nitrogens of both enol bases provide a complementary donor–acceptor arrangement, giving a relatively stable, though non‑standard, base pair that can compete with normal Watson–Crick interactions.
It has been widely accepted in nucleic acid chemistry that enol‑G prefers to pair with T (or U in RNA) rather than with C, and that this mispairing is a plausible source of transition mutations during replication. Therefore, among the given choices, the enol form of thymine (option D) provides the most compatible hydrogen-bonding geometry with the enol form of guanine.
Why the other options are wrong
Option A: Amino form of cytosine
The normal, high‑frequency base pair in DNA is keto‑G with amino‑C, not enol‑G with amino‑C. When guanine shifts to the enol tautomer, key donor and acceptor positions at N‑1, O‑6 and other ring atoms are altered, breaking the classic three‑bond pattern with amino cytosine and reducing stability.
Because the hydrogen-bond network no longer matches, amino cytosine cannot maintain a strong Watson–Crick‑like pairing with enol guanine, so this option does not represent the most stable possible partner.
Option B: Imino form of cytosine
Cytosine can undergo amino–imino tautomerism, giving a rare imino‑C with altered hydrogen‑bonding sites. Imino cytosine is known to mispair more favorably with adenine (imino‑C- A) rather than with enol‑G, because its donor–acceptor pattern better complements adenine’s imino or amino forms.
Although, in principle, some non‑canonical hydrogen bonds between enol‑G and imino‑C could form, this combination is not reported as the preferred or most stable pairing for enol guanine in theoretical and mutagenesis studies. Hence option B is incorrect.
Option C: Keto form of thymine
The keto form of thymine is the common tautomer that normally pairs with amino adenine, forming two Watson–Crick hydrogen bonds. In certain wobble situations, keto‑T can pair with G, but this interaction is weaker and typically involves distorted geometries; it is not the canonical or most stable partner for the enol form of guanine.
Theoretical analyses specifically emphasize that enol‑G pairs more strongly with enol‑T than with keto‑T because both enol tautomers present complementary donors and acceptors, whereas keto‑T’s pattern does not fully match enol‑G’s rearranged sites. Therefore, option C is less stable than option D and is not the best answer.
Key exam takeaway
For tautomer‑based mutation questions, remember these high‑yield patterns: enol‑G tends to pair with thymine (or uracil), often when both bases adopt rare enol forms, and such mispairing can generate transition mutations during DNA replication. Thus, in this question, the enol form of guanine most stably base pairs with the enol form of thymine (Option D).